Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity of each other.
In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution.
The quantitative nature of the neutralization reaction is most conveniently expressed in terms of the concentrations of acid and alkali.
For example the value of log K ≈ −6 has been estimated for hydrogen chloride in aqueous solution at room temperature.
The equation for mass-balance in hydrogen ions can then be written as where Kw represents the self-dissociation constant of water.
After multiplying both sides of the equation by [H+], it becomes and, after rearrangement and taking logarithms, With a dilute solution of the weak acid, the term 1 + TA/Ka is equal to TA/Ka to a good approximation.
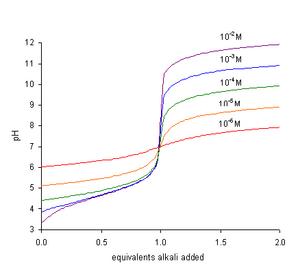
If pKw = 14, This equation explains the following facts: In a titration of a weak acid with a strong base the pH rises more steeply as the end-point is approached.
At the end-point, the slope of the curve of pH with respect to amount of titrant is a maximum.
The most suitable indicator to use for this type of titration is one, such as methyl orange, that changes color at low pH.
Chemical titration methods are used for analyzing acids or bases to determine the unknown concentration.
In wastewater treatment, chemical neutralization methods are often applied to reduce the damage that an effluent may cause upon release to the environment.
[3] Also in the digestive tract, neutralization reactions are used when food is moved from the stomach to the intestines.
In order for the nutrients to be absorbed through the intestinal wall, an alkaline environment is needed, so the pancreas produce an antacid bicarbonate to cause this transformation to occur.
To prevent the sulfur dioxide from being released, a device known as a scrubber gleans the gas from smoke stacks.
This lime then reacts with the sulfur dioxide produced forming calcium sulfite.
A suspension of lime is then injected into the mixture to produce a slurry, which removes the calcium sulfite and any remaining unreacted sulfur dioxide.