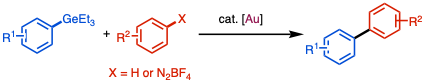Organogermanium compounds in cross-coupling reactions
[1] However, due to the toxicity, low reactivity (compared with other Ar–[M] nucleophiles) and poor stability of ArGeCl3, this reaction was demonstrated not to be synthetically applicable.
[citation needed] A stoichiometric study by Schoenebeck[2] confirmed that ArGeEt3 are inert species in the transmetallation step with Pd(II) complex.
DFT calculation indicated that the conventional concerted transmetallation mechanism has an extremely high energy barrier and not viable under the reaction conditions.
Based on this concept, if the transition metal catalyst is electron-deficient or electrophilic enough, the activation of C–Ge bond can be achievable via SEAr mechanism.
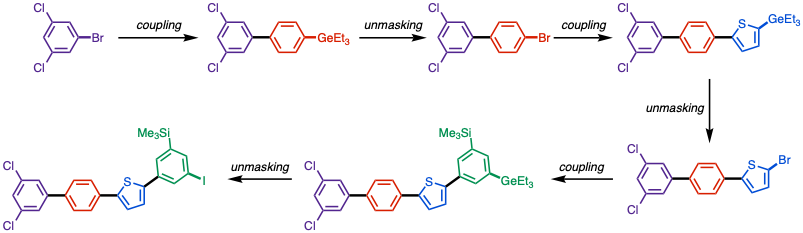
Other cross-coupling-reactive functional groups, such as other (psudo)halides, aryl boronic esters, stayed intact during the reaction.
Based on this strategy, an oxidative C–O cross-coupling method catalyzed by Pd(TFA)2 was reported.
Similarly, other reactive functional groups for cross-coupling such as (pseudo)halides and boronic esters are well tolerated.
[8] For the bromination (with NBS) and iodination (with NIS), the reaction is highly chemoselective, with halogenation solely taking place at C–Ge in presence of electron-rich arene, heterocycle substrates or other reactive functional groups.
It is also likely that there is some interaction between the nucleophilic end of the enophile and the metal in the reaction intermediate which lowers the energy of the transition state.