Uranium trioxide
Uranium trioxide is shipped between processing facilities in the form of a gel, most often from mines to conversion plants.
This alteration of uranium oxide also leads to the formation of metastudtite,[8][9] a more stable uranyl peroxide, often found in the surface of spent nuclear fuel exposed to water.
It is a poisonous, slightly radioactive substance, which may cause shortness of breath, coughing, acute arterial lesions, and changes in the chromosomes of white blood cells and gonads leading to congenital malformations if inhaled.
Solid UO3 loses O2 on heating to give green-colored U3O8: reports of the decomposition temperature in air vary from 200 to 650 °C.
Heating at 700 °C under H2 gives dark brown uranium dioxide (UO2), which is used in MOX nuclear fuel rods.
[3] While uranium trioxide is encountered as a polymeric solid under ambient conditions, some work has been done on the molecular form in the gas phase, in matrix isolations studies, and computationally.
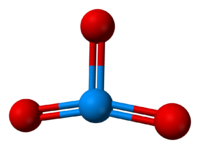
[23][24] Infrared spectroscopy of molecular UO3 isolated in an argon matrix indicates a T-shaped structure (point group C2v) for the molecule.
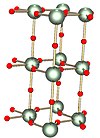
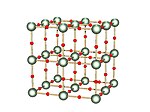
[26] The crystal structure of a uranium trioxide phase of composition UO2·82 has been determined by X-ray powder diffraction techniques using a Guinier-type focusing camera.
This is a case of a hard perhalogenated freon which is normally considered to be inert being converted chemically at a moderate temperature.
[28] Uranium trioxide can be dissolved in a mixture of tributyl phosphate and thenoyltrifluoroacetone in supercritical carbon dioxide, ultrasound was employed during the dissolution.
Dissolving uranium oxide in a strong base like sodium hydroxide forms the doubly negatively charged uranate anion (UO2−4).
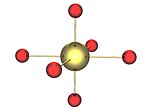
It is worth noting that uranates of the form M2UO4 do not contain UO2−4 ions, but rather flattened UO6 octahedra, containing a uranyl group and bridging oxygens.