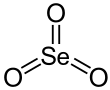
Selenium trioxide
[4] One method entails dehydration of anhydrous selenic acid with phosphorus pentoxide at 150–160 °C.
Another method is the reaction of liquid sulfur trioxide with potassium selenate.
[5] At 120 °C SeO3 reacts with selenium dioxide to form the Se(VI)-Se(IV) compound diselenium pentaoxide:[6] It reacts with selenium tetrafluoride to form selenoyl fluoride, the selenium analogue of sulfuryl fluoride As with SO3 adducts are formed with Lewis bases such as pyridine, dioxane and ether.
[4] With lithium oxide and sodium oxide it reacts to form salts of SeVIO54− and SeVIO66−:[7] With Li2O, it gives Li4SeO5, containing the trigonal pyramidal anion SeVIO54− with equatorial bonds, 170.6–171.9 pm; and longer axial Se−O bonds of 179.5 pm.
[7] SeO3 in the gas phase consists of tetramers and monomeric SeO3 which is trigonal planar with an Se−O bond length of 168.78 pm.