Peptide bond
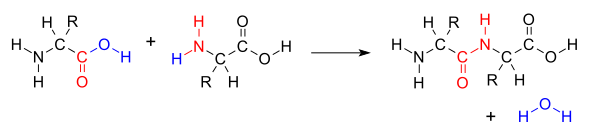
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain.
When two amino acids form a dipeptide through a peptide bond,[1] it is a type of condensation reaction.
This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−).
[10] In living organisms, the process is normally catalyzed by enzymes known as peptidases or proteases, although there are reports of peptide bond hydrolysis caused by conformational strain as the peptide/protein folds into the native structure.
The wavelength of absorption for a peptide bond is 190–230 nm,[12] which makes it particularly susceptible to UV radiation.
Significant delocalisation of the lone pair of electrons on the nitrogen atom gives the group a partial double-bond character.
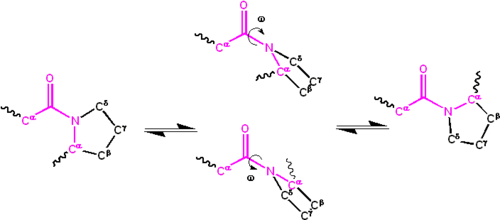
The partial double bond renders the amide group planar, occurring in either the cis or trans isomers.
However, X-Pro peptide groups tend to have a roughly 30:1 ratio, presumably because the symmetry between the Cα and Cδ atoms of proline makes the cis and trans isomers nearly equal in energy, as shown in the figure below.
Both of these mechanisms for lowering the activation energy have been observed in peptidyl prolyl isomerases (PPIases), which are naturally occurring enzymes that catalyze the cis-trans isomerization of X-Pro peptide bonds.
Due to its resonance stabilization, the peptide bond is relatively unreactive under physiological conditions, even less than similar compounds such as esters.
When the functional group attacking the peptide bond is a thiol, hydroxyl or amine, the resulting molecule may be called a cyclol or, more specifically, a thiacyclol, an oxacyclol or an azacyclol, respectively.