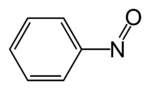
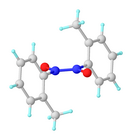
Nitrosobenzene
Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer.
The monomeric material is selectively sublimed due to its lower molecular weight and is collected on a cold finger as lustrous, dark green crystals.
Over time, the monomeric material dimerizes to give the parent azobenzene dioxide as a pale yellow solid.
[2] Nitrosobenzene was first prepared by Adolf von Baeyer by the reaction of diphenylmercury and nitrosyl bromide:[4] A modern synthesis entails reduction of nitrobenzene to phenylhydroxylamine (C6H5NHOH) which is then oxidized by sodium dichromate (Na2Cr2O7).
Most characteristically, nitrosobenzene condenses with active methylene groups, such as those of malonic esters and phenylacetonitrile.