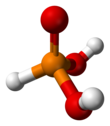
Phosphorous acid
This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula.
In common with other phosphorus oxides with P−H bonds (e.g.hypophosphorous acid and dialkyl phosphites),[2] it exists in equilibrium with an extremely minor tautomer P(OH)3.
Chemistry examinations often test students' appreciation of the fact that not all three hydrogen atoms are acidic under aqueous conditions, in contrast with H3PO4.
[5] Both phosphorous acid and its deprotonated forms are good reducing agents, although not necessarily quick to react.
This nomenclature is commonly reserved for substituted derivatives, that is, organic group bonded to phosphorus, not simply an ester.