Phosphoric acids and phosphates
In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron.
Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures.
Indeed, the term phosphoric acid often means this compound specifically (and this is also the current IUPAC nomenclature).
Polyphosphoric acids are used in organic synthesis for cyclizations and acylations; an alternative is Eaton's reagent.
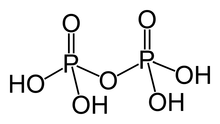
[1][2][3] Metaphosphoric acid (HPO3) is a colorless, vitreous, deliquescent solid, density 2.2 to 2.5 g/cc, which sublimes upon heating.
[4] Phosphoric acid units can be bonded together in rings (cyclic structures).
Removal of the hydrogen atoms as protons H+ turns a phosphoric acid into a phosphate anion.
Likewise, tripolyphosphoric acid H5P3O10 yields at least five anions [H5−kP3O10]k−, where k ranges from 1 to 5, including tripolyphosphate [P3O10]5−.
The general formula for such (non-cyclic) polyphosphate anions, linear or branched, is [Hn+2−kPnO3n+1]k−, where the charge k may vary from 1 to n + 2.
A general formula for such cyclic compounds is [HPO3]x where x = number of phosphoric units in the molecule.
When metaphosphoric acids lose their hydrogens as H+, cyclic anions called metaphosphates are formed.
Higher temperature or acidic conditions can speed up the hydrolysis reactions considerably.
Ortho-, pyro-, and tripolyphosphate compounds have been commonly used in detergents (i. e. cleaners) formulations.
are called diphosphate, triphosphate, tetraphosphate, etc., especially when they are part of phosphate esters in biochemistry.
[6] As a corrosion inhibitor, polyphosphates work by forming a protective film on the interior surface of pipes.
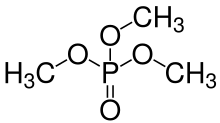
Since orthophosphoric acid has three −OH groups, it can esterify with one, two, or three alcohol molecules to form a mono-, di-, or triester.
See the general structure image of an ortho- (or mono-) phosphate ester below on the left, where any of the R groups can be a hydrogen or an organic radical.
Any −OH groups on the phosphates in these ester molecules may lose H+ ions to form anions, again depending on the pH in a solution.
In the biochemistry of living organisms, there are many kinds of (mono)phosphate, diphosphate, and triphosphate compounds (essentially esters), many of which play a significant role in metabolism such as adenosine diphosphate (ADP) and triphosphate (ATP).