Transition metal pincer complex
Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration.
This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end.
Although the most common class of pincer ligands features PCP donor sets, variations have been developed where the phosphines are replaced by thioethers and tertiary amines.
Many pincer ligands also feature nitrogenous donors at the central coordinating group position (see figure), such as pyridines.
Terpy and its relatives lack the steric bulk of the two terminal donor sites found in traditional pincer ligands.
[5][6] Ni(II) N,N,N pincer complexes are active in Kumada, Sonogashira, and Suzuki-Miyaura coupling reactions with unactivated alkyl halides.
[9] Due to the firm tridentate coordination mode, it allows the metal complexes to exhibit high thermal stability as well as air-stability.
Changing the hardness/softness of the donor, using electron-withdrawing groups (EWGs) in the backbone, and the altering the steric constraints of the ligands are all methods used to tune the reactivity at the metal centre.
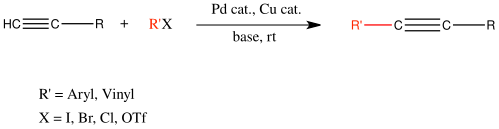
[13] Using PCP pincer-palladium catalysts, aryl-aryl couplings can be achieved with turnover numbers (TONs) upwards of 900,000 and high yields.
[5] Additionally, other groups have found that very low catalyst loadings can be achieved with asymmetric palladium pincer complexes.
This rate difference may be due to the availability of the Ir(V) oxidation state which allows stronger Ir-C and Ir-H bonds.
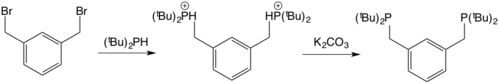
[16] [17] The original work on PCP ligands arose from studies of the Pt(II) complexes derived from long-chain ditertiary phosphines, species of the type R2P(CH2)nPR2 where n >4 and R = tert-butyl.