RNA interference
Andrew Fire and Craig Mello shared the 2006 Nobel Prize in Physiology or Medicine for their work on RNAi in the nematode worm Caenorhabditis elegans, which they published in 1998.
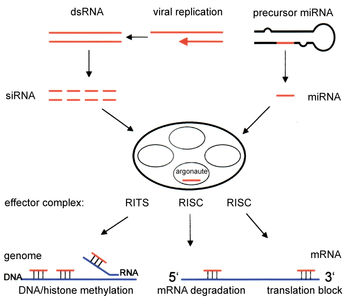
RNAi has an important role in defending cells against parasitic nucleotide sequences (e.g., viruses or transposons) and also influences development of organisms.
Specifically, this is accomplished when the guide strand pairs with a complementary sequence in a mRNA molecule and induces cleavage by Ago2, a catalytic component of the RISC.
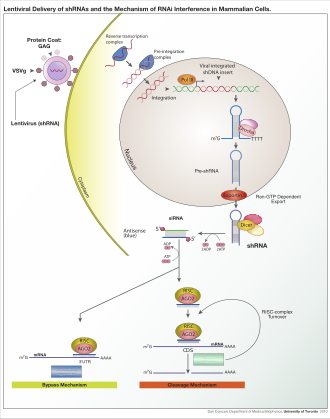
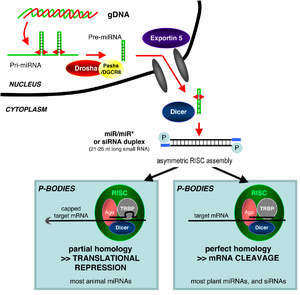
[7] Exogenous dsRNA initiates RNAi by activating the ribonuclease protein Dicer,[8] which binds and cleaves dsRNAs in plants, or short hairpin RNAs (shRNAs) in humans, to produce double-stranded fragments of 20–25 base pairs with a 2-nucleotide overhang at the 3′ end.
[9] Bioinformatics studies on the genomes of multiple organisms suggest this length maximizes target-gene specificity and minimizes non-specific effects.
A miRNA is expressed from a much longer RNA-coding gene as a primary transcript known as a pri-miRNA which is processed, in the cell nucleus, to a 70-nucleotide stem-loop structure called a pre-miRNA by the microprocessor complex.
By binding to specific sites within the 3′-UTR, miRNAs can decrease gene expression of various mRNAs by either inhibiting translation or directly causing degradation of the transcript.
[17] The active components of an RNA-induced silencing complex (RISC) are endonucleases called Argonaute proteins, which cleave the target mRNA strand complementary to their bound siRNA.
[52] Indeed, deletion of these genes in the fission yeast S. pombe disrupts histone methylation and centromere formation,[53] causing slow or stalled anaphase during cell division.
The formation of such a heterochromatin region, though not its maintenance, is Dicer-dependent, presumably because Dicer is required to generate the initial complement of siRNAs that target subsequent transcripts.
[56] Heterochromatin maintenance has been suggested to function as a self-reinforcing feedback loop, as new siRNAs are formed from the occasional nascent transcripts by RdRP for incorporation into local RITS complexes.
[57] The relevance of observations from fission yeast mating-type regions and centromeres to mammals is not clear, as heterochromatin maintenance in mammalian cells may be independent of the components of the RNAi pathway.
[63] Further support for this model comes from studies on ADAR-null C. elegans strains indicating that A→I RNA editing may counteract RNAi silencing of endogenous genes and transgenes.
[83][84] A similar role in immunity may operate in C. elegans, as Argonaute proteins are upregulated in response to viruses and worms that overexpress components of the RNAi pathway are resistant to viral infection.
A large-scale comparative genomics study likewise indicates that the eukaryotic crown group already possessed these components, which may then have had closer functional associations with generalized RNA degradation systems such as the exosome.
[100] This study also suggests that the RNA-binding Argonaute protein family, which is shared among eukaryotes, most archaea, and at least some bacteria (such as Aquifex aeolicus), is homologous to and originally evolved from components of the translation initiation system.
[101][102] Extensive efforts in computational biology have been directed toward the design of successful dsRNA reagents that maximize gene knockdown but minimize "off-target" effects.
[10] A multitude of software tools have been developed implementing algorithms for the design of general[103][104] mammal-specific,[105] and virus-specific[106] siRNAs that are automatically checked for possible cross-reactivity.
Breaking down ALAS1 mRNA prevents toxins (responsible for neurovisceral attacks and AHP disease) such as aminolevulinic acid (ALA) and porphobilinogen (PBG) from accumulating.
In response to these potential issues and barriers, two approaches help facilitate siRNA delivery to target cells: lipid nanoparticles and conjugates.
In December 2020, Novartis announced that positive results from phase III efficacy studies deemed inclisiran was a treatment for heterozygous familial hypercholesterolemia (HeFH) and atherosclerotic cardiovascular disease (ASCVD).
Numerous studies have demonstrated that RNAi can provide a more specific approach to inhibit tumor growth by targeting cancer-related genes (i.e., oncogene).
Studies in cells and in mouse have shown that specifically targeting Amyloid beta-producing genes (e.g. BACE1 and APP) by RNAi can significantly reduced the amount of Aβ peptide which is correlated with the cause of Alzheimer's disease.
The use of the RNAi pathway has developed numerous products such as foods like Arctic apples, nicotine-free tobacco, decaffeinated coffee, nutrient fortified vegetation and hypoallergenic crops.
Cotton seeds are rich in dietary protein but naturally contain the toxic terpenoid product gossypol, making them unsuitable for human consumption.
[176] Animals exposed to RNAi at doses millions of times higher than anticipated human exposure levels show no adverse effects.
[178] Drosophila spp., Bombyx mori, Locusta spp., Spodoptera spp., Tribolium castaneum, Nilaparvata lugens, Helicoverpa armigera, and Apis mellifera are models that have been widely used to learn how RNAi works within particular taxa of insects.
Further investigation of the phenomenon in plants indicated that the downregulation was due to post-transcriptional inhibition of gene expression via an increased rate of mRNA degradation.
[198] Craig C. Mello and Andrew Fire's 1998 Nature paper reported a potent gene silencing effect after injecting double stranded RNA into C.
[201] Only a year later, McCaffrey and colleagues demonstrated that this sequence-specific silencing had therapeutic applications by targeting a sequence from the Hepatitis C virus in transgenic mice.