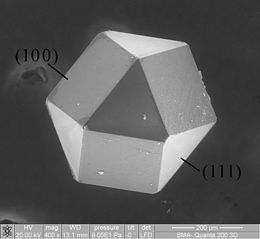
Solid
Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas.
[4] Some solids, particularly most organic compounds, are held together with van der Waals forces resulting from the polarization of the electronic charge cloud on each molecule.
The strength and reliability of metals has led to their widespread use in construction of buildings and other structures, as well as in most vehicles, many appliances and tools, pipes, road signs and railroad tracks.
The study of metallic elements and their alloys makes up a significant portion of the fields of solid-state chemistry, physics, materials science and engineering.
More advanced models of metal properties consider the effect of the positive ions cores on the delocalised electrons.
The vast majority of the rocks of the Earth's crust consist of quartz (crystalline SiO2), feldspar, mica, chlorite, kaolin, calcite, epidote, olivine, augite, hornblende, magnetite, hematite, limonite and a few other minerals.
Brittle fracture is very characteristic of most ceramic and glass-ceramic materials that typically exhibit low (and inconsistent) values of KIc.
For an example of applications of ceramics, the extreme hardness of zirconia is utilized in the manufacture of knife blades, as well as other industrial cutting tools.
Ceramic engines do not require a cooling system and hence allow a major weight reduction and therefore greater fuel efficiency.
Turbine engines made with ceramics could operate more efficiently, giving aircraft greater range and payload for a set amount of fuel.
Glass-ceramics are used to make cookware (originally known by the brand name CorningWare) and stovetops that have high resistance to thermal shock and extremely low permeability to liquids.
Glass ceramics may also occur naturally when lightning strikes the crystalline (e.g. quartz) grains found in most beach sand.
In this case, the extreme and immediate heat of the lightning (~2500 °C) creates hollow, branching rootlike structures called fulgurite via fusion.
Organic chemistry studies the structure, properties, composition, reactions, and preparation by synthesis (or other means) of chemical compounds of carbon and hydrogen, which may contain any number of other elements such as nitrogen, oxygen and the halogens: fluorine, chlorine, bromine and iodine.
Examples of organic solids include wood, paraffin wax, naphthalene and a wide variety of polymers and plastics.
In proteins, these differences give the polymer the ability to adopt a biologically active conformation in preference to others (see self-assembly).
Polymers that have been around, and that are in current widespread use, include carbon-based polyethylene, polypropylene, polyvinyl chloride, polystyrene, nylons, polyesters, acrylics, polyurethane, and polycarbonates, and silicon-based silicones.
Applications of composite materials range from structural elements such as steel-reinforced concrete, to the thermally insulative tiles that play a key and integral role in NASA's Space Shuttle thermal protection system, which is used to protect the surface of the shuttle from the heat of re-entry into the Earth's atmosphere.
One example is Reinforced Carbon-Carbon (RCC), the light gray material that withstands reentry temperatures up to 1,510 °C (2,750 °F) and protects the nose cap and leading edges of Space Shuttle's wings.
In order to provide oxidation resistance for reuse capability, the outer layers of the RCC are converted to silicon carbide.
Silicon nanowires cycle without significant degradation and present the potential for use in batteries with greatly expanded storage times.
Here again, surface area of the nanoparticles (and thin films) plays a critical role in maximizing the amount of absorbed radiation.
Physical properties of elements and compounds that provide conclusive evidence of chemical composition include odor, color, volume, density (mass per unit volume), melting point, boiling point, heat capacity, physical form and shape at room temperature (solid, liquid or gas; cubic, trigonal crystals, etc.
For example, steel beams are used in construction because of their high strength, meaning that they neither break nor bend significantly under the applied load.
For example, glass-ceramics have become extremely useful for countertop cooking, as they exhibit excellent mechanical properties and can sustain repeated and quick temperature changes up to 1000 °C.
The spectrum of lattice vibrations in a crystalline or glassy network provides the foundation for the kinetic theory of solids.
When voltage is applied to the capacitor, electric charges of equal magnitude, but opposite polarity, build up on each plate.
The deformation (~0.1%) lends itself to useful technical applications such as high-voltage sources, loudspeakers, lasers, as well as chemical, biological, and acousto-optic sensors and/or transducers.
Fundamentally, the device needs to fulfill only two functions: photo-generation of charge carriers (electrons and holes) in a light-absorbing material, and separation of the charge carriers to a conductive contact that will transmit the electricity (simply put, carrying electrons off through a metal contact into an external circuit).
The intellectual origins of materials science stem from the Age of Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy.