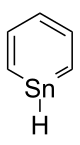
Stannabenzene
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom.
Stannabenzene itself has been studied by computational chemistry,[1] but has not been isolated.
The 2-stannanaphthalene depicted below is stable in an inert atmosphere at temperatures below 140 °C.
[2] The tin to carbon bond in this compound is shielded from potential reactants by two very bulky groups, one tert-butyl group and the even larger 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl or Tbt group.
[4] At room-temperature it quantitatively forms the DA dimer.