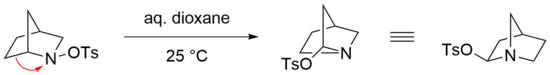Stieglitz rearrangement
[4][14] In a Beckmann rearrangement, the acid catalyzed carbon to nitrogen migration takes place on the oxime to yield a nitrilium ion intermediate.
[4] However, after the generation of the positively charged iminium ion through the π-interaction between the nitrogen lone pair and the electron deficient carbon in the Stieglitz rearrangement, the pathways diverge.
Alternatively, if the starting material did not possess any amino protons, the neutral state can be achieved with an external reducing agent, such as sodium borohydride.
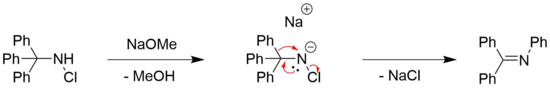
[17] Although the original Stieglitz reaction is best known for the rearrangement of trityl hydroxylamines, there are several variations which include good leaving groups as N-substituents (such as halogens and sulfonates).
[18] The generated intermediate can then undergo rearrangement by the migration of the phenyl group and dissociation of the phosphorus(V) species to form N-phenyl benzophenone imine.
[20] In the latter case, BF3 acts as a Lewis acid in the electrophilic activation of the benzylic oxygen to allow for a nucleophilic attack on the adjacent nitrogen atom.
[22] The Stieglitz rearrangement is especially reactive in the case of bridged bicyclic N-sulfonated amines as starting materials, where mild conditions are sufficient for an efficient reaction to take place.
[24] However, the respective imine is not formed in this case, presumably due to the strain that would thermodynamically disfavor such a structure, bearing a double bond at a bridgehead atom (Bredt's rule).
An alternative way for the production of protonated organic azides is the nuclophilic addition of hydrazoic acid to a carbocations, which can then also undergo Stieglitz rearrangements.
An example for that can be found in the total synthesis of (±)-lycopodine by Paul Grieco et al.[6][29] There, a ring formation takes place by a rearrangement on a secondary haloamine by subjecting it to silver tetrafluoroborate.