Sulfate
[3][4] A widely accepted description involving pπ – dπ bonding was initially proposed by Durward William John Cruickshank.
[6] In this model, the structure obeys the octet rule and the charge distribution is in agreement with the electronegativity of the atoms.
However, Pauling's representation for sulfate and other main group compounds with oxygen is still a common way of representing the bonding in many textbooks.
Alum, a double sulfate of potassium and aluminium with the formula K2Al2(SO4)4·24H2O, figured in the development of the chemical industry.
Sulfates occur as microscopic particles (aerosols) resulting from fossil fuel and biomass combustion.
The anaerobic sulfate-reducing bacteria Desulfovibrio desulfuricans and D. vulgaris can remove the black sulfate crust that often tarnishes buildings.
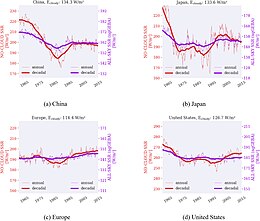
[19][20][21][22][23] This followed measures taken to combat air pollution by the developed nations, typically through flue-gas desulfurization installations at thermal power plants, such as wet scrubbers or fluidized bed combustion.
[24][25][26] In the United States, sulfate aerosols have declined significantly since 1970 with the passage of the Clean Air Act, which was strengthened in 1977 and 1990.
[27] By 2010, this reduction in sulfate pollution led to estimated healthcare cost savings valued at $50 billion annually.
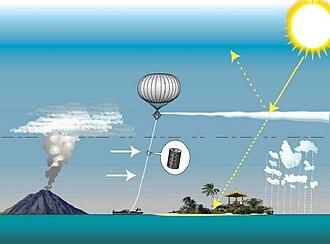
[29] Since changes in aerosol concentrations already have an impact on the global climate, they would necessarily influence future projections as well.
In fact, it is impossible to fully estimate the warming impact of all greenhouse gases without accounting for the counteracting cooling from aerosols.
[35] On regional and global scale, air pollution can affect the water cycle, in a manner similar to some natural processes.
[43] Likewise, it has been suggested since the early 2000s that since aerosols decrease solar radiation over the ocean and hence reduce evaporation from it, they would be "spinning down the hydrological cycle of the planet.




1 with polar covalent bonds only; 2 with an ionic bond