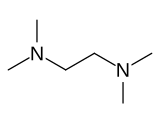
Tetramethylethylenediamine
This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups.
[5] In such complexes [Li(tmeda)2]+ behaves like a quaternary ammonium salt, such as [NEt4]+.
sec-Butyllithium/TMEDA is a useful combination in organic synthesis where the n-butyl analogue adds to substrate.
TMEDA is still capable of forming a metal complex with Li in this case as mentioned above.
TEMED is a common reagent in molecular biology laboratories, as a polymerizing agent for polyacrylamide gels in the protein analysis technique SDS-PAGE.