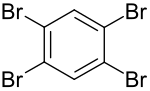
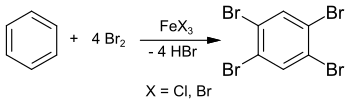
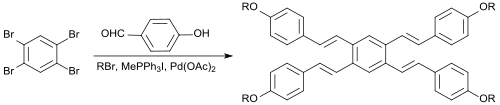
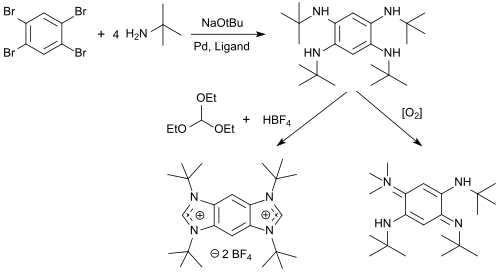
1,2,4,5-Tetrabromobenzene
In his 1885 dissertation, Adolf Scheufelen published the synthesis of a purer sample using iron(III) chloride FeCl3 as a catalyst, isolated as "pretty needles" ("schönen Nadeln").
In a one-pot reaction, 1,2,4,5-tetrabromobenzene reacts with 4-hydroxybenzaldehyde, the alkylating agent 1-bromopentane, the Wittig reagent methyltriphenylphosphonium iodide, the base potassium carbonate, the phase transfer catalyst tetrabutylammonium bromide, the Heck reagent palladium(II)acetate and the Heck co-catalyst 1,3-bis(diphenylphosphino)propane (dppp) in dimethylacetamide obtaining directly a symmetrical tetraalkoxylstilbene as E-isomer in 17% yield.
[9] Due to their pronounced π-conjugation such compounds could be potentially applied as optical brighteners, OLED materials or liquid crystals.
[15] If the dibromene oxide is allowed to react further with furan, in the presence of n-butyllithium[12] or potassium amide[16] or via an intermediate 1,4-aryne the tricyclic 1,4-adduct 1,4:5.8-diepoxy-1,4,5,8-tetrahydroanthracene[17] is formed in 71% yield as a syn-anti-mixture.
[18] The crosslinking of benzimidazole-modified polymers provides materials with a high absorption capacity for carbon dioxide, which could be suitable for CO2 separation from gas mixtures.