Tetrahedral carbonyl addition compound
[3] The first evidence for tetrahedral intermediates in the substitution reactions of carboxylic derivatives was provided by Myron L. Bender in 1951.
At the end of the reaction he found that the remaining starting material had a decreased proportion of labeled oxygen, which is consistent with the existence of the tetrahedral intermediate.
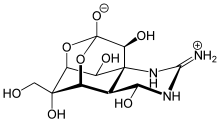
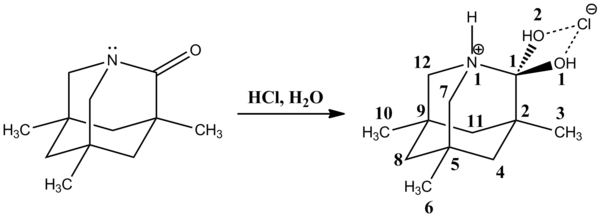
In 2002 David Evans et al. observed a very stable neutral tetrahedral intermediate in the reaction of N-acylpyrroles with organometallic compounds, followed by protonation with ammonium chloride producing a carbinol.
The elongated C1-N1, and shortened C1-O1 bonds are explained with an anomeric effect resulting from the interaction of the oxygen lone pairs with the σ*C-N orbital.
So the addition to the carbonyl group allows some of the strain inherent in the small ring to be released, which is why cyclopropanone and cyclobutanone are very reactive electrophiles.
For larger rings, where the bond angles are not as distorted, the stability of the hemiacetals is due to entropy and the proximity of the nucleophile to the carbonyl group.
In contrast, the formation of cyclic hemiacetals involves a single molecule reacting with itself, making the reaction more favorable.
Another way to understand the stability of cyclic hemiacetals is to look at the equilibrium constant as the ratio of the forward and backward reaction rate.
In the presence of acid, hemiacetals can undergo an elimination reaction, losing the oxygen atom that once belonged to the parent aldehyde’s carbonyl group.
These oxonium ions are powerful electrophiles, and react rapidly with a second molecule of alcohol to form new, stable compounds, called acetals.
Acetals, as already pointed out, are stable tetrahedral intermediates so they can be used as protective groups in organic synthesis.
Quantum mechanical calculations have shown that the tetrahedral adduct is formed easily and it is fairly stable, in agreement with the experimental results.
These proteins have evolved to recognize and bind the transition state of peptide hydrolysis reaction which is a tetrahedral intermediate.
Therefore, the main protease inhibitors are tetrahedral intermediate mimics having an alcohol or a phosphate group.
In the mammalian serine proteases, trypsin and chymotrypsin, two peptide NH groups of the polypeptide backbone form the so-called oxyanion hole by donating hydrogen bonds to the negatively charged oxygen atom of the tetrahedral intermediate.