Thiosulfate
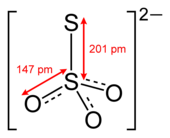
Thiosulfate (IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula S2O2−3.
This view precluded the disproportionation reaction of thiosulfate into sulfate and sulfide as a redox mechanism for providing energy to bacteria under anaerobic conditions in sediments because there is no change in oxidation state for either S atom.
This observation is consistent with the disproportionation of thiosulfate into sulfate and sulfide as a redox mechanism freeing up energy from microbial fermentation.
[citation needed] In the era of silver-based photography, thiosulfate salts were consumed on a large scale as a "fixer" reagent.
Sodium thiosulfate, commonly called hypo (from "hyposulfite"), was widely used in photography to fix black and white negatives and prints after the developing stage; modern "rapid" fixers use ammonium thiosulfate as a fixing salt because it acts three to four times faster.
[5] Thiosulfate salts have been used to extract or leach gold and silver from their ores as a less toxic alternative to cyanide ion.
It is most effective in a pre-hospital setting, since immediate administration by emergency personnel is necessary to reverse rapid intracellular hypoxia caused by the inhibition of cellular respiration, at complex IV.
[10][11] Thiosulfate can also work as electron donor for growth of bacteria oxidizing sulfur, such as Chlorobium limicola forma thiosulfatophilum.