Transition state analog
Kinetic isotope effect (KIE) is a measurement of the reaction rate of isotope-labeled reactants against the more common natural substrate.
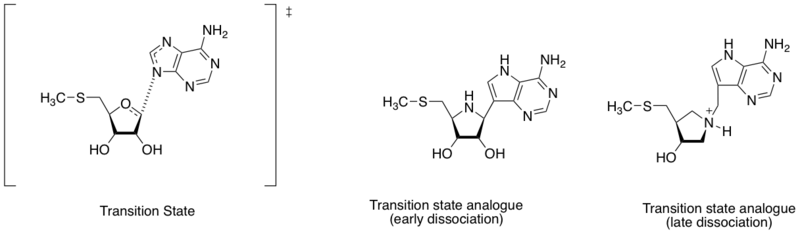
[7] Given the different distance between nitrogen atom of adenine and the ribose anomeric carbon (see in the diagram in this section), the transition state structure can be defined by early or late dissociation stage.
[8] Thermolysin is an enzyme produced by Bacillus thermoproteolyticus that catalyses the hydrolysis of peptides containing hydrophobic amino acids.
The water molecule is then activated by both the zinc ion and the Glu143 residue and attacks the carbonyl carbon to form a tetrahedral transition state (see figure).
Holden and coworkers then mimicked that tetrahedral transition state to design a series of phosphonamidate peptide analogues.
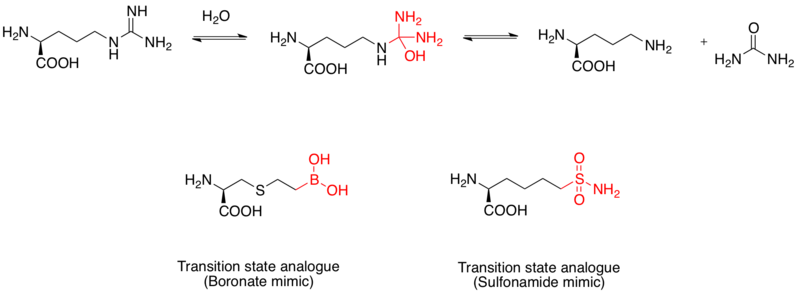
[11] The mechanism of hydrolysis of L-arginine is carried out via nucleophilic attack on the guanidino group by water, forming a tetrahedral intermediate.
[12] Evidence of boronic acid mimics as transition state analogue inhibitors of human arginase I was elucidated by x-ray crystal structures.