Metalloprotein
In metalloproteins, metal ions are usually coordinated by nitrogen, oxygen or sulfur centers belonging to amino acid residues of the protein.
Given the diversity of the metalloproteome, virtually all amino acid residues have been shown to bind metal centers.
[10] In addition to donor groups that are provided by amino acid residues, many organic cofactors function as ligands.
These are the second stage product of protein hydrolysis obtained by treatment with slightly stronger acids and alkalies.
Hemoglobin, which is the principal oxygen-carrier in humans, has four subunits in which the iron(II) ion is coordinated by the planar macrocyclic ligand protoporphyrin IX (PIX) and the imidazole nitrogen atom of a histidine residue.
[11] This change in spin state is a cooperative effect due to the higher crystal field splitting and smaller ionic radius of Fe2+ in the oxyhemoglobin moiety.
The iron atoms are coordinated to the protein through the carboxylate side chains of a glutamate and aspartate and five histidine residues.
The uptake of O2 by hemerythrin is accompanied by two-electron oxidation of the reduced binuclear center to produce bound peroxide (OOH−).
[14][15] Chlorocruorin (as the larger carrier erythrocruorin) is an oxygen-binding hemeprotein present in the blood plasma of many annelids, particularly certain marine polychaetes.
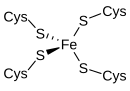
The active site contains an iron ion coordinated by the sulfur atoms of four cysteine residues forming an almost regular tetrahedron.
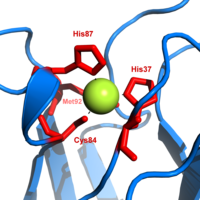
[20] The trigonal plane of the pyramidal base is composed of two nitrogen atoms (N1 and N2) from separate histidines and a sulfur (S1) from a cysteine.
The elongated Cu−S2 bonding destabilizes the Cu(II) form and increases the redox potential of the protein.
The blue color (597 nm peak absorption) is due to the Cu−S1 bond where S(pπ) to Cu(dx2−y2) charge transfer occurs.
Iron is transported by transferrin whose binding site consists of two tyrosines, one aspartic acid and one histidine.
Metalloenzymes all have one feature in common, namely that the metal ion is bound to the protein with one labile coordination site.
The positively-charged zinc ion polarizes the coordinated water molecule, and nucleophilic attack by the negatively-charged hydroxide portion on carbon dioxide proceeds rapidly.
[27] The cobalt-containing Vitamin B12 (also known as cobalamin) catalyzes the transfer of methyl (−CH3) groups between two molecules, which involves the breaking of C−C bonds, a process that is energetically expensive in organic reactions.
[28] The structure of the coenzyme was famously determined by Dorothy Hodgkin and co-workers, for which she received a Nobel Prize in Chemistry.
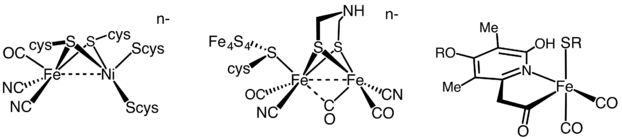
There are three components to its action: a molybdenum atom at the active site, iron–sulfur clusters that are involved in transporting the electrons needed to reduce the nitrogen, and an abundant energy source in the form of magnesium ATP.
It appears to contain a MoFe7S8 cluster that is able to bind the dinitrogen molecule and, presumably, enable the reduction process to begin.
These properties render the superoxide ion very toxic and are deployed to advantage by phagocytes to kill invading microorganisms.
In solutions at neutral pH, the superoxide ion disproportionates to molecular oxygen and hydrogen peroxide.
This enzyme also contains zinc ions for stabilization and is activated by copper chaperone for superoxide dismutase (CCS).
This species is, in effect, a free radical, and is very reactive and allows an electron to be transferred to acceptors that are adjacent to the chlorophyll in the chloroplast.
This reduction ultimately draws electrons from water, yielding molecular oxygen as a final oxidation product.
[37] Many ribozymes require metal ions in their active sites for chemical catalysis; hence they are called metalloenzymes.
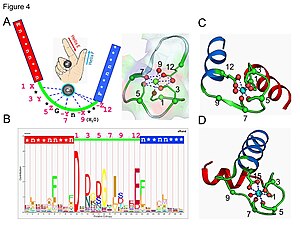
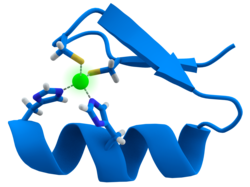
In an EF-hand loop protein domain, the calcium ion is coordinated in a pentagonal bipyramidal configuration.
Calmodulin participates in an intracellular signaling system by acting as a diffusible second messenger to the initial stimuli.
[46][47] In both cardiac and skeletal muscles, muscular force production is controlled primarily by changes in the intracellular calcium concentration.
Troponin, along with actin and tropomyosin, is the protein complex to which calcium binds to trigger the production of muscular force.