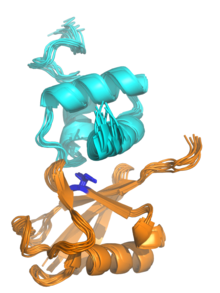Ubiquitin-binding domain
Many UBDs can be roughly classified into four broad categories: alpha-helical structures (in some cases as small as a single helix, as in the ubiquitin-interacting motif); zinc fingers; pleckstrin homology (PH) domains; and domains similar to those in ubiquitin-conjugating (also known as E2) enzymes.
[8] Zinc finger UBDs have a broader range of binding modes including interactions with polar residues.
[5] Most UBDs described to date bind to monoubiquitin and thus do not show a linkage-preference for the differently linked ubiquitin chains.
There are, however, a handful of known, linkage-specific UBDs, that can specifically differentiate between the eight different ubiquitin linkages.
This is important as the different linkage types are thought to signal for different molecular processes and linkage-specific recognition of these chains ensures the appropriate cellular response.