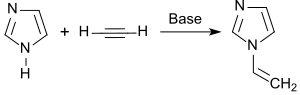
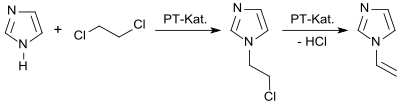
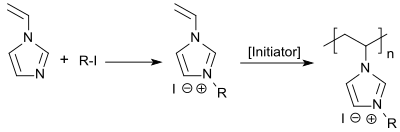
1-Vinylimidazole
[3] Another lab scale procedure reports the vinylation of imidazole with bromoethene and kieselguhr-supported cesium fluoride in acetonitrile with a yield of 65%.
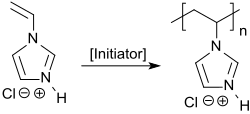
[6] The resulting quaternary ammonium compounds can be free-radically polymerized in aqueous solution with the water-soluble azo initiator 4,4'-azobisvaleric acid.
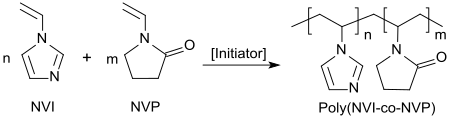
Copolymers of quaternary N-vinylimidazolium salts and polar monomers (in particular N-vinylpyrrolidone) are cationic polyelectrolytes and are suitable, inter alia, as flocculants for water treatment, as flotation auxiliaries for coal and ore processing, as additives for drilling fluids and cementations in the extraction of oil, as emulsion cleavers for the dewatering of crude oil emulsions in refineries, and as corrosion inhibitors for iron alloys.
[8] 1-Vinylimidazole polymerizes radically in an aqueous or alcoholic solution to form homopolymers with average molar masses of from 2,000 to 50,000,[9] which, however, often still contain relatively high residual monomer contents (> 600 ppm).
[10] By adding sulfur-containing chain regulators, such as mercaptoethanol, the undesired residual content of the N-vinylimidazole can be reduced to less than 50 ppm, although the molar mass of the polymer obtained also decreases.