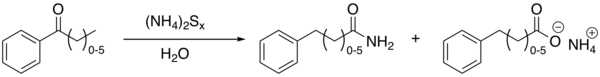
Willgerodt rearrangement
[1][2][3][4] The formation of the corresponding carboxylic acid is a side reaction resulting from hydrolysis of the amide.
The net effect is thus migration of the carbonyl group to the end of the chain and oxidation.
An example with modified reagents (sulfur, concentrated ammonium hydroxide and pyridine) is the conversion of acetophenone to 2-phenylacetamide and phenylacetic acid[5] The related Willgerodt–Kindler reaction[6] takes place with elemental sulfur and an amine like morpholine.
This reacts as a nucleophile with electrophilic sulfur, similar to an Stork enamine alkylation reaction.
[verification needed] The actual rearrangement reaction takes place when the amine group attacks the thiocarbonyl in a nucleophilic addition temporarily forming an aziridine and the thioacetamide by tautomerization.