Alcian blue stain
74240, formerly called Alcian blue 8GX from the name of a batch of an ICI product) has been historically the most common and the most reliable member.
[1] It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc.
Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue (DMB or DMMB) method.
The Monstral blue found to coat the inside of copper vessels used to process phthalic acid derivatives had led to the discovery of Phthalocyanine in 1907.
[7] While the popularity of Alcian blue expanded exponentially, the difficulty involved in its production due to environmentally hazardous intermediate steps made its availability difficult and ICI stopped producing it by 1973.
Prof J. E. Scott worked to decipher the chemistry of Alcian blue, which was known only to the Industry but kept as a tight trade secret.
[8] After the interim crisis since the 1970s when ICI had to stop, there have now been environmentally safe alternative industrial manufacturing of this dye that is supposed to work as well as 8GX but is called 8G since it is made differently.
[9] In attempt to answer what was the importance of discovering an alternative method of manufacturing this compound, a company (Anatech Ltd, USA) that remanufactured Alcian blue says: The etymology of the name is not certain, and whether to capitalize it is an editorial style choice.
[12] Oxford online dictionary mentions that it was a trademark and also specifies[13] This hypothesis is consistent with the name of Alcian green, which is a tetraphenyl-phthalocyanine with copper.
[14] However Prof. J. E. Scott who had cracked the chemistry of Alcian blue himself and later received confirmation from the manufacturer (ICI) wrote that Alcian was a trademark that ICI preferred to be spelt starting with a capital "A", and he presumes it came from the old English word "halcyon", which has a "romantic and poetic associations with the kingfisher bird and calm seas".
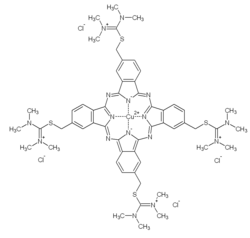
[16] The solid Alcian blue is obtained as greenish-black (or sometimes dark bluish violet[17]) crystals with metallic sheen.
It is a tetravalent basic (cationic) dye with a copper (Cu2+, coordination 4 of 6, orbital configuration d9 with Jahn–Teller distortion) phthalocyanine nucleus (CuPc) with three or four pendent isothiouronium side chains imparting its bulkiness and positive charges.
These groups split off from the macrocyclic ring during the washing at the end of staining or by rather mild conditions (e.g. pH above 5.6) or during spontaneous degradation.
[22] Nucleic acids are generally basophilic because they have a very high density of negative charge due to the sugar phosphate backbone.
However, in contrast to other basic i.e. cationic dyes, Alcian blue usually (given the right pH and salt concentrations, and normal temperature and duration in minutes, not hours) preferably stains acidic glycosaminoglycans but not the chromatin and nissel substance, the mechanism of which had been a mystery for a long time and various theories were proposed.
Though the presumed basis of the staining is its positive charge attracted to negative structures (e.g. acidic sugars), bulkiness (width 2.5–3 nm, compared to toluidine blue ~0.7 x1.1 nm[23]) makes its diffusion very slow in less permeable parts of the tissue and thus prevent it from staining highly negative yet compact structures such as chromatin and nissl substance.
ICI sold thousands of tons of alcian blue and filed multiple patents regarding its manufacturing process to keep its chemistry a tight secret.
Alcian blue has been used as an adhesive to help stick glycol methacrylate sections to glass slides (which have negatively charged silicate groups).
[27] Alcian blue carries a large aromatic surface that can participate in van der Waals interactions, as well as multiple localized charges.
Alcian blue coated surfaces hold onto the negatively charged glycocalyx so tightly that it can even be used to cover a layer of cells and then float it up to peel off the roof ("unroofing") to study the cytoplasmic side of the plasma membrane.
[28] Alcian Blue has been used as a gelling agent for lubricating fluids likely due to the stacking properties of this macrocylic aromatic compound.