Applied spectroscopy
Applied spectroscopy is the application of various spectroscopic methods for the detection and identification of different elements or compounds to solve problems in fields like forensics, medicine, the oil industry, atmospheric chemistry, and pharmacology.
The point of attack occurs at the tertiary carbon atom present in every repeat unit, causing oxidation and finally chain breakage.
The branch points are tertiary carbon atoms, so polymer degradation starts there and results in chain cleavage, and embrittlement.
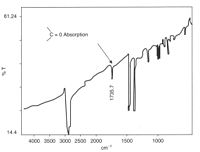
In the example shown at left, carbonyl groups were readily detected by IR spectroscopy from a cast thin film.
Many process methods such as extrusion and injection moulding involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken.
The crutch had fractured across a polypropylene insert within the aluminium tube of the device, and IR spectroscopy of the material showed that it had oxidised, possibly as a result of poor moulding.
The carbonyl group can be further oxidised to break the chain, so weakening the material by lowering the molecular weight, and cracks start to grow in the regions affected.
For example, two EDX spectra were obtained during an investigation into ozone cracking of diaphragm seals in a semiconductor fabrication factory.