Aryl halide
The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.
Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals.
[3] Arenes with electron donating groups react with halogens even in the absence of Lewis acids.
For example, phenols and anilines react quickly with chlorine and bromine water to give multihalogenated products.
The oxychlorination of benzene has been well investigated, motivated by the avoidance of HCl as a coproduct in the direct halogenation:[3] This technology is not widely used however.
The main aryl bromides produced commercially are tetrabromophthalic anhydride, decabromodiphenyl ether, and tetrabromobisphenol-A.
Synthetic aryl iodides are used as X-ray contrast agents, but otherwise these compounds are not produced on a large scale.
[11] Synthetic T4 is one of the most heavily prescribed medicines in the U.S.[12] Many chlorinated and brominated aromatic compounds are produced by marine organisms.
Aryl halides with electron-withdrawing groups in the ortho and para positions, can undergo SNAr reactions.
[16] A 2018 paper indicates that this situation may actually be rather common, occurring in systems that were previously assumed to proceed via SNAr mechanisms.
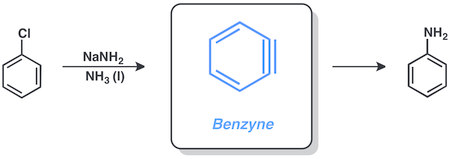
Chlorobenzene and sodium amide react in liquid ammonia to give aniline by this pathway.
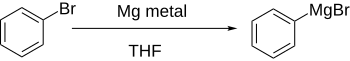
Illustrative is the preparation of phenyllithium from bromobenzene using n-butyllithium (n-BuLi): Direct formation of Grignard reagents, by adding the magnesium to the aryl halide in an ethereal solution, works well if the aromatic ring is not significantly deactivated by electron-withdrawing groups.
Alternatively aryl halides, especially the bromides and iodides, undergo oxidative addition, and thus are subject to Buchwald–Hartwig amination-type reactions.
Overall, production of aryl chlorides (also naphthyl derivatives) has been declining since the 1980s, in part due to environmental concerns.