Babler oxidation
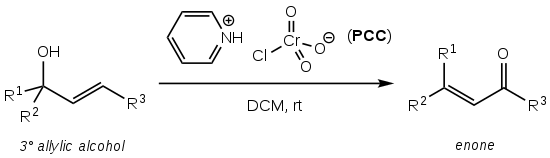
The Babler oxidation, also known as the Babler-Dauben oxidation, is an organic reaction for the oxidative transposition of tertiary allylic alcohols to enones using pyridinium chlorochromate (PCC):[1] It is named after James Babler who first reported the reaction in 1976[1][2] and William Dauben who extended the scope to cyclic systems in 1977, thereby significantly increasing the synthetic utility:[1][3] The reaction produces the desired enone product to high yield (typically >75%), is operationally simple and does not require air-free techniques or heating.
[1] It suffers, however, from the very high toxicity and environmental hazard posed by the hexavalent chromium PCC oxidising reagent.
[1][4] The reaction proceeds through the formation of a chromate ester (1) from nucleophilic attack of the chlorochromate by the allylic alcohol.
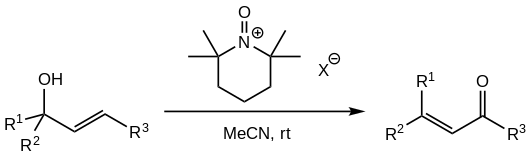
Commonly reported stoichiometric reagents for this purpose include di-tert-butyl peroxide, 2-iodoxybenzoic acid or periodates.
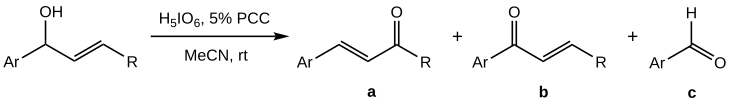
[1] The Babler-Dauben oxidation of secondary allylic alcohols proves more difficult to control than that of tertiary analogues, as along with the desired product (a) a mixture with high proportion of side-products (b) and (c) is obtained:[1] The yield of a is found to be maximised when PCC is not used in stoichiometric quantities but as a co-oxidant; the best effect (50–70% yield of a) is achieved for orthoperiodic acid as the main oxidiser with a 5% molar PCC.