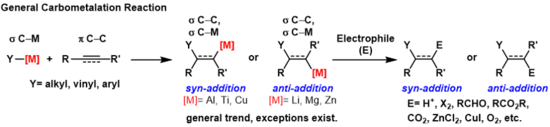
Carbometalation
Carbometallations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively.
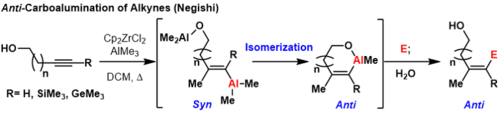
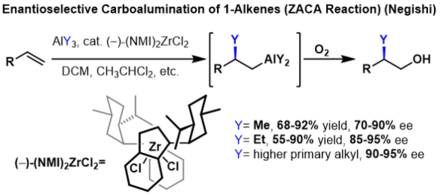
[1] This reaction is sometimes referred to as the Zr- catalyzed asymmetric carboalumination of alkenes (ZACA) or the Zr-catalyzed methylalumination of alkynes (ZMA).
This zirconium cation can coordinate an alkene or alkyne where migratory insertion of a methyl then takes place.
The resultant vinyl or alkyl zirconium species can undergo a reversible, but stereoretentive transmetalation with an organoaluminium to provide the carboalumination product and regeneration of the zirconocene dichloride catalyst.
However, recent advances in the synthesis of sparteine surrogates and their effective application in carbolithiation have reactivated interest in this strategy.
[8] Another demonstration of this reaction type is an alternative route to tamoxifen starting from diphenylacetylene and ethyllithium:[9] The capturing electrophile here is triisopropyl borate forming the boronic acid R–B(OH)2.
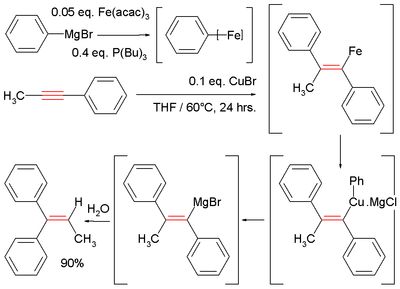
These reactions can be catalyzed by a variety of transition metals such as iron,[10][11] copper,[10] zirconium,[12] nickel,[10][13] cobalt[14] and others.
Illustrative is the Fe-catalyzed reaction of methylphenylacetylene with phenylmagnesium bromide, which generates a vinyl magnesium intermediate.