Metal tetranorbornyl
Computational calculations have determined that London dispersion effects significantly contribute to the stability of metal tetranorbornyls.
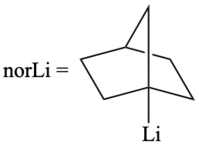
[3] Traditionally, metal tetranorbornyls are prepared by a reaction of alkyllithiums, such as 1-norbornyllithium, with transition-metal halides while tumbling with glass beads in pentane.
The use of the alumina column allows for the collection of a purple fraction that undergoes solvent evaporation and sublimation to obtain the desired Cr(nor)4 complex.
[1][3] Quantum mechanical calculations have elucidated that London dispersion forces between the norbornyl ligands are accountable for the stability and molecular geometry of the homoleptic tetranorbornyl metal complexes.
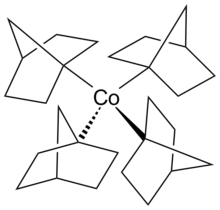
When examined by x-ray crystallography, the metal tetranorbornyl has a crystallographic Cs symmetry due to the presence of six carbons laid on the mirror plane.
However, the four carbons atoms bonded to the cobalt metal center resembled a tetragonally compressed tetrahedron, which appeared as a pseudo D2d symmetry.
The single unpaired electron resides in the antibonding t2 orbital, which would cause the structure to experience a Jahn-Teller distortion.
However, Theopold and co-workers speculated that the slight tetragonal compression could have been a result of steric interactions between norbornyl ligands and crystal packing forces.
[1] The 1-norbornyl ligands on the complex have a strong dispersion attraction and high ring strain, which as a consequence hinders the α- and β-hydride elimination reactions.
[1] A combination of London dispersion force and steric effects from the 1-norbornyl ligands results in the stability observed for the metal center.
[3] The resulting molecular geometry of the metal tetranorbornyls complexes is due to the unpaired and paired d electrons.
Tetrakis(1-norbornyl)molybdenum was observed as a room temperature EPR signal that originated from a d2 metal center, which was considered to have two unpaired electrons in the eg orbital.
[6][13] In 1988, Klaus H. Theopold and Erin K. Byrne performed the electrochemical experiment, cyclic voltammetry, to determine how oxidizing was the metal center of the tetrakis(1-norbornyl)cobalt(IV) complex.