Methylation
Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function.
This process, catalyzed by such enzymes as caffeoyl-CoA O-methyltransferase, is a key reaction in the biosynthesis of lignols, percursors to lignin, a major structural component of plants.
Plants produce flavonoids and isoflavones with methylations on hydroxyl groups, i.e. methoxy bonds.
The most prevalent protein methylations affect arginine and lysine residue of specific histones.
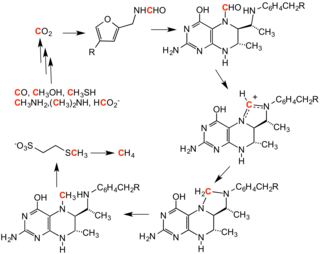
The overall reaction transforms 5-methyltetrahydrofolate (N5-MeTHF) into tetrahydrofolate (THF) while transferring a methyl group to Hcy to form Met.
The activated methyl group is transferred from Me-Cob to the Hcy thiolate, which regenerates Co(I) in Cob, and Met is released from the enzyme.
[5] Biomethylation is the pathway for converting some heavy elements into more mobile or more lethal derivatives that can enter the food chain.
Improper methylations of human genes can lead to disease development,[10][11] including cancer.
[12][13] In honey bees, DNA methylation is associated with alternative splicing and gene regulation based on functional genomic research published in 2013.
[14] In addition, DNA methylation is associated with expression changes in immune genes when honey bees were under lethal viral infection.
[20] In social insects such as honey bees, RNA methylation is studied as a possible epigenetic mechanism underlying aggression via reciprocal crosses.
Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.
[25] Indeed, pharmacological inhibition of global methylation in species ranging from human, mouse, fish, fly, roundworm, plant, algae, and cyanobacteria causes the same effects on their biological rhythms, demonstrating conserved physiological roles of methylation during evolution.
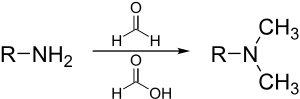
[26] The term methylation in organic chemistry refers to the alkylation process used to describe the delivery of a CH3 group.
Strongly nucleophilic methylating agents include methyllithium (CH3Li)[38] or Grignard reagents such as methylmagnesium bromide (CH3MgX).