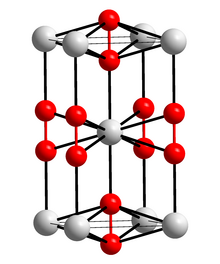
Caesium superoxide
It consists of caesium cations Cs+ and superoxide anions O−2.
[2] Caesium superoxide's crystal structure is same as calcium carbide.
[2] It reacts with water to form hydrogen peroxide and caesium hydroxide.
[2] Heating to approximately 400 °C induces thermal decomposition to caesium peroxide.
[3] The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol.