Carbon suboxide
1.114 g/cm3, liquid[2] Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula C3O2 and structure O=C=C=C=O.
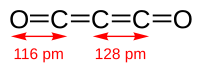
Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.
The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current.
[11] Carbon suboxide is used in the preparation of malonates; and as an auxiliary to improve the dye affinity of furs.
It has been shown that carbon suboxide in an organism can quickly polymerize into macrocyclic polycarbon structures with the common formula (C3O2)n (mostly (C3O2)6 and (C3O2)8), and that those macrocyclic compounds are potent inhibitors of Na+/K+-ATP-ase and Ca-dependent ATP-ase, and have digoxin-like physiological properties and natriuretic and antihypertensive actions.
[13][14][15] Other than that, some authors think also that those macrocyclic compounds of carbon suboxide can possibly diminish free radical formation and oxidative stress and play a role in endogenous anticancer protective mechanisms, for example in the retina.
Studies generally agree that the molecule is highly non-rigid, with a very shallow barrier to bending.
According to one study, the molecular geometry is described by a double-well potential with a minimum at θC2 ~ 160°, an inversion barrier of 20 cm−1 (0.057 kcal/mol), and a total energy change of 80 cm−1 (0.23 kcal/mol) for 140° ≤ θC2 ≤ 180°.
[17] The small energetic barrier to bending is around the same order of magnitude as the vibrational zero-point energy.
While infrared[18] and electron diffraction[19] studies have indicated that C3O2 has a bent structure in the gas phase, the compound was found to possess at least an average linear geometry in the solid phase by X-ray crystallography, although the large thermal ellipsoids of the oxygen atoms and C2 have been interpreted to be consistent with rapid bending (minimum θC2 ~ 170°), even in the solid state.
[10] A heterocumulene resonance form of carbon suboxide based on minimization of formal charges does not readily explain the molecule's non-rigidity and deviation from linearity.