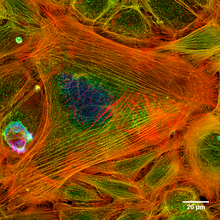
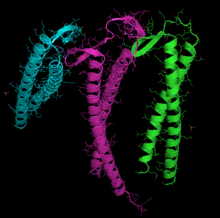
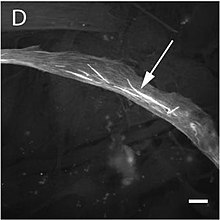
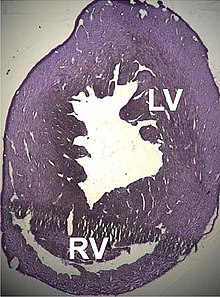
Actin
Other enzymes or organelles such as cilia can be anchored to this scaffolding in order to control the deformation of the external cell membrane, which allows endocytosis and cytokinesis.
Mutations in the different genes that regulate actin production in humans can cause muscular diseases, variations in the size and function of the heart as well as deafness.
[18] Authors describe a protein present in the nuclear fraction, obtained from Xenopus laevis oocytes, which shows the same features as skeletal muscle actin.
[62] In addition to the physical force generated by actin polymerization, microfilaments facilitate the movement of various intracellular components by serving as the roadway along which a family of motor proteins called myosins travel.
The resulting spike in cytosolic calcium rapidly releases tropomyosin and troponin from the actin thread, allowing myosin to bind, and muscle contracation to begin.
[67] In the fission yeast Schizosaccharomyces pombe, actin is actively formed in the constricting ring with the participation of Arp3, the formin Cdc12, profilin, and WASp, along with preformed microfilaments.
Once the ring has been constructed the structure is maintained by a continual assembly and disassembly that, aided by the Arp2/3 complex and formins, is key to one of the central processes of cytokinesis.
[85] The X-ray crystallography model of actin that was produced by Kabsch from the striated muscle tissue of rabbits is the most commonly used in structural studies as it was the first to be purified.
[86][87] Elzinga and co-workers first determined the complete peptide sequence for this type of actin in 1973, with later work by the same author adding further detail to the model.
[94] The terms "pointed" and "barbed" referring to the two ends of the microfilaments derive from their appearance under transmission electron microscopy when samples are examined following a preparation technique called "decoration".
[97] During the resting phase the tropomyosin covers the actin's active sites so that the actin-myosin interaction cannot take place and produce muscular contraction.
The reason for this special route could be the need to avoid the presence of incorrectly folded actin monomers, which could be toxic as they can act as inefficient polymerization terminators.
It was initially thought that it only bound with actin and tubulin, although recent immunoprecipitation studies have shown that it interacts with a large number of polypeptides, which possibly function as substrates.
The actin is recognized, loaded, and delivered to the cytosolic chaperonin (CCT) in an open conformation by the inner end of prefoldin's "tentacles" (see the image and note).
Its function is to bind the water molecule that produces a nucleophilic attack on the ATP's γ-phosphate bond, while the nucleotide is strongly bound to subdomains 3 and 4.
[94][97] The "open" to "closed" transformation between G and F forms and its implications on the relative motion of several key residues and the formation of water wires have been characterized in molecular dynamics and QM/MM simulations.
For example, the protein that is coded by the ACTG2 gene in humans is completely equivalent to the homologues present in rats and mice, even though at a nucleotide level the similarity decreases to 92%.
[134] Bacteria therefore possess a cytoskeleton with homologous elements to actin (for example, MreB, AlfA, ParM, FtsA, and MamK), even though the amino acid sequence of these proteins diverges from that present in animal cells.
[144] The most common symptoms consist of a typical facial morphology (myopathic facies), muscular weakness, a delay in motor development and respiratory difficulties.
[155][156] Two cases of dilated cardiomyopathy have been studied involving a substitution of highly conserved amino acids belonging to the protein domains that bind and intersperse with the Z discs.
[160] Recent studies have discovered ACTC1 mutations that are implicated in two other pathological processes: Infantile idiopathic restrictive cardiomyopathy,[161] and noncompaction of the left ventricular myocardium.
A number of pseudogenes exist that are distributed throughout the genome, and its sequence contains six exons that can give rise to up to 21 different transcriptions by alternative splicing, which are known as the β-actins.
Consistent with this complexity, its products are also found in a number of locations and they form part of a wide variety of processes (cytoskeleton, NuA4 histone-acyltransferase complex, cell nucleus) and in addition they are associated with the mechanisms of a great number of pathological processes (carcinomas, juvenile dystonia, infection mechanisms, nervous system malformations and tumour invasion, among others).
[164] Three pathological processes have so far been discovered that are caused by a direct alteration in gene sequence: The ACTG1 locus codes for the cytosolic γ-actin protein that is responsible for the formation of cytoskeletal microfilaments.
It contains six exons, giving rise to 22 different mRNAs, which produce four complete isoforms whose form of expression is probably dependent on the type of tissue they are found in.
[170] In terms of pathology, it has been associated with processes such as amyloidosis, retinitis pigmentosa, infection mechanisms, kidney diseases, and various types of congenital hearing loss.
[171] However, although there is no record of any case, it is known that γ-actin is also expressed in skeletal muscles, and although it is present in small quantities, model organisms have shown that its absence can give rise to myopathies.
Two basic forms are present in bacteria: In addition to the previously cited example, actin polymerization is stimulated in the initial steps of the internalization of some viruses, notably HIV, by, for example, inactivating the cofilin complex.
[190] However, Halliburton was unable to further refine his findings, and the discovery of actin is credited instead to Brunó Ferenc Straub, a young biochemist working in Albert Szent-Györgyi's laboratory at the Institute of Medical Chemistry at the University of Szeged, Hungary.
Other approaches such as the use of cryo-electron microscopy and synchrotron radiation have recently allowed increasing resolution and better understanding of the nature of the interactions and conformational changes implicated in the formation of actin filaments.