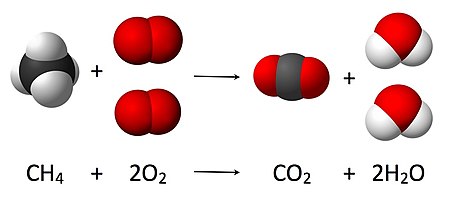
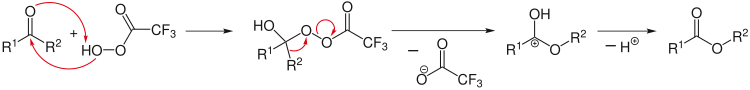
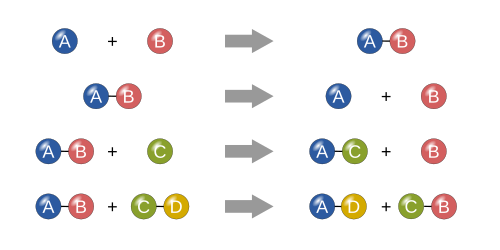
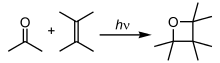
Chemical reaction
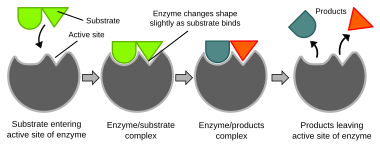
Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperature and concentrations present within a cell.
[7] From the 16th century, researchers including Jan Baptist van Helmont, Robert Boyle, and Isaac Newton tried to establish theories of experimentally observed chemical transformations.
This proved to be false in 1785 by Antoine Lavoisier who found the correct explanation of the combustion as a reaction with oxygen from the air.
Based on this idea and the atomic theory of John Dalton, Joseph Proust had developed the law of definite proportions, which later resulted in the concepts of stoichiometry and chemical equations.
Other chemists who brought major contributions to organic chemistry include Alexander William Williamson with his synthesis of ethers and Christopher Kelk Ingold, who, among many discoveries, established the mechanisms of substitution reactions.
Here the analysis starts from the products, for example by splitting selected chemical bonds, to arrive at plausible initial reagents.
A typical example of a unimolecular reaction is the cis–trans isomerization, in which the cis-form of a compound converts to the trans-form or vice versa.
The time to reach equilibrium depends on parameters such as temperature, pressure, and the materials involved, and is determined by the minimum free energy.
For example, an increase in pressure due to decreasing volume causes the reaction to shift to the side with fewer moles of gas.
Typical examples of exothermic reactions are combustion, precipitation and crystallization, in which ordered solids are formed from disordered gaseous or liquid phases.
According to Le Chatelier's Principle, reactions may proceed in the forward or reverse direction until they end or reach equilibrium.
is positive, which means that if they occur at constant temperature and pressure, they increase the Gibbs free energy of the reaction.
[23][24] Examples include: In a combustion reaction, an element or compound reacts with an oxidant, usually oxygen, often producing energy in the form of heat or light.
Conversely, the sodium is oxidized or is the electron donor, and thus induces a reduction in the other species and is considered the reducing agent.
Elements try to reach the low-energy noble gas configuration, and therefore alkali metals and halogens will donate and accept one electron, respectively.
This is achieved by providing lone pairs of the ligand into empty orbitals of the metal atom and forming dipolar bonds.
It usually takes place when the concentration of dissolved ions exceeds the solubility limit[34] and forms an insoluble salt.
Rapid precipitation results in an amorphous or microcrystalline residue and a slow process can yield single crystals.
In photochemical reactions, atoms and molecules absorb energy (photons) of the illumination light and convert it into an excited state.
The premier example is photosynthesis, in which most plants use solar energy to convert carbon dioxide and water into glucose, disposing of oxygen as a side-product.
In heterogeneous catalysis, typical secondary processes include coking where the catalyst becomes covered by polymeric side products.
Acids are an example of a homogeneous catalyst, they increase the nucleophilicity of carbonyls, allowing a reaction that would not otherwise proceed with electrophiles.
One of the most industrially important reactions is the cracking of heavy hydrocarbons at oil refineries to create smaller, simpler molecules.
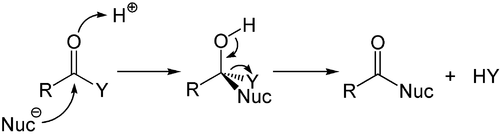
In their names, S stands for substitution, N for nucleophilic, and the number represents the kinetic order of the reaction, unimolecular or bimolecular.
[49] The addition and its counterpart, the elimination, are reactions that change the number of substituents on the carbon atom, and form or cleave multiple bonds.
In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group.
The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule.
Among the most important of its mechanisms is the anabolism, in which different DNA and enzyme-controlled processes result in the production of large molecules such as proteins and carbohydrates from smaller units.
Important energy sources are glucose and oxygen, which can be produced by plants via photosynthesis or assimilated from food and air, respectively.
It is essential to make the reaction as efficient as possible, maximizing the yield and minimizing the number of reagents, energy inputs and waste.