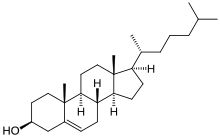
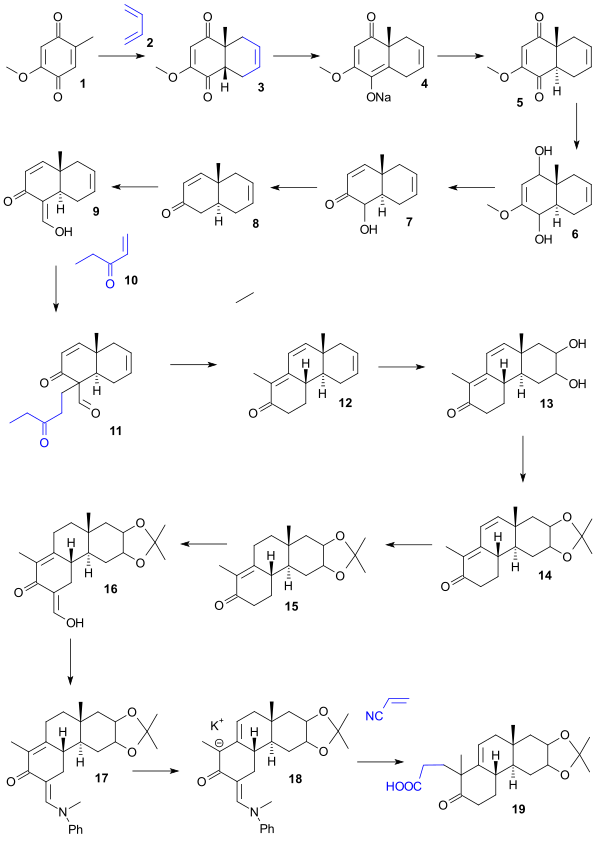
Cholesterol total synthesis
[1] The research group of Robert Robinson with John Cornforth (Oxford University) published their synthesis in 1951 [2] and that of Robert Burns Woodward with Franz Sondheimer (Harvard University) in 1952.
Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
The conversion of cholestanol to cholesterol was already demonstrated by oxidation of the ketone, bromination to the bromoketone and elimination to the enone.
The conversion of cholestenone into cholesterol by the method of Dauben and Eastham (1950) [10] consisted of reduction of the enol acetate (lithium aluminium hydride) and fractionation with digitonin for the isolation of the correct isomer.
Starting point for the Woodward synthesis was the hydroquinone 1 that was converted to cis-bicycle 2 in a Diels-Alder reaction with butadiene.
Treatment with periodic acid (dioxane) and piperidine acetate (benzene) gave aldehyde 24 through diol 22 (oxidation) and dialdehyde 23 (aldol condensation).