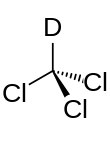
Deuterated chloroform
The large difference in boiling points between the starting material and product facilitate purification by distillation.
In carbon-13 NMR spectroscopy, the sole carbon in deuterated chloroform shows a triplet at a chemical shift of 77.16 ppm with the three peaks being about equal size, resulting from splitting by spin coupling to the attached spin-1 deuterium atom (CHCl3 has a chemical shift of 77.36 ppm).
[4] Deuterated chloroform is a general purpose NMR solvent, as it is not very chemically reactive and unlikely to exchange its deuterium with its solute,[9] and its low boiling point allows for easy sample recovery.
Chloroform reacts photochemically with oxygen to form chlorine, phosgene and hydrogen chloride.
To slow this process and reduce the acidity of the solvent, chloroform-d is stored in brown-tinted bottles, often over copper chips or silver foil as stabilizer.