Fischer projection
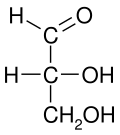
Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry.
The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers.
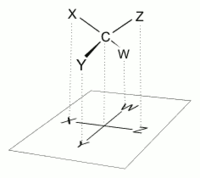
To do so, all attachments to main chain carbons must be rotated such that resulting Newman projections show an eclipsed configuration.
However, when creating a Fischer projection for a monosaccharide with more than three carbons, there is no way to orient the molecule in space so that all horizontal bonds will be slanted toward the viewer.
It can be regarded as a projection of a modified version of the molecule, ideally twisted at multiple levels along its backbone.
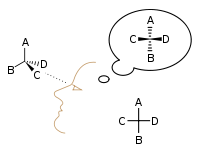
A great benefit of the model is the ability to interpret chirality with ease based on the orientation of the substituents.
The primary difference is the benefit that Fischer Projections provide in depicting the orientation of substituents with the vertical and horizontal lines.
In certain cases, it can be helpful to draw a Fischer Projection from a larger molecule to visualize and determine the chirality of a specific carbon.
Newman projections are another system that can be used as they showcase the structure of a molecule in the staggered or eclipsed conformation states.