Gran plot
Such plots have been also used to calibrate glass electrodes, to estimate the carbonate content of aqueous solutions, and to estimate the Ka values (acid dissociation constants) of weak acids and bases from titration data.
[1] Gran plots use linear approximations of the a priori non-linear relationships between the measured quantity, pH or electromotive potential (emf), and the titrant volume.
Other types of concentration measures, such as spectrophotometric absorbances or NMR chemical shifts, can in principle be similarly treated.
The graphing and visual estimation of the end point have been replaced by more accurate least-squares analyses since the advent of modern computers and enabling software packages, especially spreadsheet programs with built-in least-squares functionality.
In a titration of strong acid with strong alkali, the analytical concentration of the hydrogen ion is obtained from the initial concentration of acid, Ci and the amount of alkali added during titration.
If E0 and s are known from electrode calibration, where the line crosses the x-axis indicates the volume at the equivalence point,
Alternatively, this plot can be used for electrode calibration by finding the values of E0 and s that give the best straight line.
Otherwise, a relatively high concentration of background electrolyte can be used, or the activity quotient can be computed.
[2] Mirror-image plots are obtained if titrating the base with the acid, and the signs of the slopes are reversed.
Hence, Figure 1 gives sample Gran plots of a strong base-strong acid titration.
The method can be used to estimate the dissociation constants of weak acids, as well as their concentrations (Gran, 1952).
Figure 2 gives an example; in this example, the two x-intercepts differ by about 0.2 mL but this is a small discrepancy, given the large equivalence volume (0.5% error).
Similar equations can be written for the titration of a weak base by strong acid (Gran, 1952; Harris, 1998).
Martell and Motekaitis (1992) use the most linear regions and exploit the difference in equivalence volumes between acid-side and base-side plots during an acid-base titration to estimate the adventitious CO2 content in the base solution.
In that situation, the extra acid used to neutralize the carbonate, by double protonation, in volume
In the opposite case of a titration of acid by base, the carbonate content is similarly computed from
When the total CO2 content is significant, as in natural waters and alkaline effluents, two or three inflections can be seen in the pH-volume curves owing to buffering by higher concentrations of bicarbonate and carbonate.
As discussed by Stumm and Morgan (1981), the analysis of such waters can use up to six Gran plots from a single titration to estimate the multiple end points and measure the total alkalinity and the carbonate and/or bicarbonate contents.
If a pH electrode is not well calibrated, an offset correction can be computed in situ from the acid-side Gran slope: In the sample data illustrated in Figure 1, this offset correction was not insignificant, at -0.054 pH units.
For instance, Martell and Motekaitis (1992) calculated the pH value expected at the start of the titration, having earlier titrated the acid and base solutions against primary standards, then adjusted the pH electrode reading accordingly, but this does not afford a slope correction if one is needed.
Based on earlier work by McBryde (1969), Gans and O'Sullivan (2000) describe an iterative approach to arrive at both
This program additionally can compute (by a separate, non-linear least-squares process) a 'correction' for the base concentration.
Note that the regular Gran functions will provide the required equivalence volumes and, as
in step 1 can be had from the slope of the regular acid-side Gran function as detailed earlier.
Note too that this procedure computes the CO2 content and can indeed be combined with a complete standardization of the base, using the definition of
The significance of the slopes will depend on the interactions between the two species, whether associating in solution or precipitating together (Gran, 1952).
In any titration lacking buffering components, both before-equivalence and beyond-equivalence plots should ideally cross the x axis at the same point.
Non-ideal behaviour can result from measurement errors (e.g. a poorly calibrated electrode, an insufficient equilibration time before recording the electrode reading, drifts in ionic strength), sampling errors (e.g. low data densities in the linear regions) or an incomplete chemical model (e.g. the presence of titratable impurities such as carbonate in the base, or incomplete precipitation in potentiometric titrations of dilute solutions, for which Gran et al. (1981) propose alternate approaches).
terms in the Gran functions only asymptotically tend toward, and never reach, the x axis, curvature approaching the equivalence point is to be expected in all cases.
In the sample plots displayed in the Figures, the most linear regions (the data represented by filled circles) were selected for the least-squares computations of slopes and intercepts.

Figure 1. Sample Gran plots using data from Butler (1998). Note that the filled circles indicate the data points included in the least-squares computations to give the fitted dashed lines.
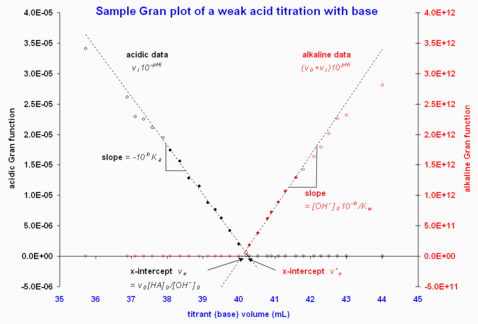
Figure 2. Sample Gran plots using data from "an on-line source" . Retrieved 2008-02-18 . Only the region near equivalence is shown, as data far from equivalence deviate strongly from linearity. Note that the filled circles indicate the data points included in the least-squares computations to give the fitted dashed lines.

Figure 3. Sample Gran plots using data from a titration of Cl − by Ag + monitored potentiometrically. The potentials were converted to [Ag + ] values for plotting. Note that the filled circles indicate the data points included in the least-squares computations to give the fitted dashed lines.