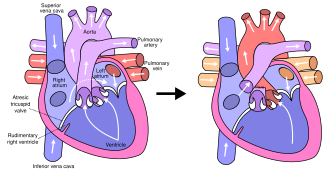
Hypoplastic left heart syndrome
[5] Initial management is geared to maintaining patency of the ductus arteriosus - a connection between the pulmonary artery and the aorta that closes shortly after birth.
If left untreated, patients with HLHS die within the first weeks of life while 70% of those that undergo three-staged palliative surgery reach adulthood.
After surgery, children with HLHS typically experience neurodevelopmental as well as motor delay and are at an increased risk of heart failure as adults.
Closing of the ductus arteriosus in a heart that is severely underdeveloped on the left results in cyanosis and respiratory distress which can progress to cardiogenic shock and death.
[5] In neonates with a small atrial septal defect, termed "restrictive", there is inadequate mixing of oxygenated and deoxygenated blood.
[16][17] Possible contributing factors may include intrauterine infarction, infectious changes, and a selective left ventricular cardiomyopathy.
[15][18] A popular theory termed the "no flow, no grow" hypothesis suggest that primary anatomic defects of the aortic and mitral valves lead to malformations of the left ventricle and its outflow tract.
[9] These primary defects can be divided into those that lead to outflow tract obstruction or reduced left ventricular filling.
Aortic stenosis that occurs during fetal development results in added stress on the left ventricle in utero.
[5] In typical anatomy, the left side of the heart receives oxygen-rich blood from the lungs and pumps it to the rest of the body.
Patients with HLHS can have a number of cardiac malformations that ultimately lead to a diminutive left ventricle that is unable to supply sufficient blood flow to the rest of the body.
[20] In the mitral stenosis and aortic atresia (MS-AA) subtype blood is able to fill the left ventricle, however it is unable to be supplied to the systemic circulation via the hypoplastic ascending aorta.
[5] Chest x-ray may also be utilized in the diagnosis of hypoplastic left heart syndrome, and typically shows an enlarged cardiac silhouette along with signs pulmonary hypertension.
Genetic testing may be beneficial to obtain and has been associated with multiple chromosomal abnormalities including Turner, DiGeorge, and Down syndrome.
[23][24] Surgical operations to assist with hypoplastic left heart are complex and need to be individualized for each patient.
[18] Many studies show that the higher the volume (number of surgeries performed) at a hospital, the lower the mortality (death) rate.
[25][26] Factors that increase an infant's risk include lower birth weight, additional congenital anomalies, a genetic syndrome or those with a highly restrictive atrial septum.
[28] Current research focuses on charting the connections between neurodevelopment injuries, surgical and intensive care procedures, and genetic susceptibility with the goal of modifying interventions that impair neurodevelopmental and psychosocial outcomes.
However, due to the vast improvement of surgical intervention, with many hospitals achieving over 90% survival, there is debate on whether or not compassionate care should still be offered to families.
In addition, both the Blalock-Taussig and the Sano shunts expose the lungs to systemic arterial pressures, leading to long-term pulmonary hypertension and, eventually, heart failure.
[33] Although there are several variations, the functional effect is to redirect venous blood from the lower body (through the inferior vena cava) away from the right atrium to the pulmonary artery.
[18] Prognosis is dependent upon the health of the child, as there is an increased demand on respiratory and heart rate in infants during common childhood illnesses.
[41] Children with HLHS and other comparable single-ventricle conditions, as a group, have poorer neurodevelopmental outcomes than their healthy peers.
[45] A systematic review found 23 articles, published since 2010, as well as nine relevant clinical trials related to congenital heart disease and recent advances in stem cell therapies.
The first use of autologous umbilical cord blood cells was done at the Mayo Clinic in 2015 and was found to increase right ventricular function in the patient after their procedure.
[46] There are several ongoing studies testing the feasibility and efficacy of stem cell therapies for single ventricle diseases such as HLHS.