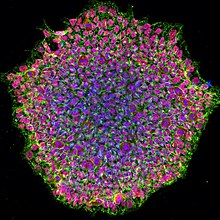
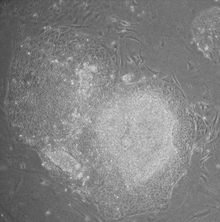
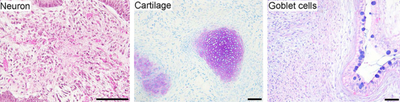
Induced pluripotent stem cell
[1] Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent.
However, since the generation of embryonic stem cells involves destruction (or at least manipulation)[4] of the pre-implantation stage embryo, there has been much controversy surrounding their use.
[6][7][8][9] In his Nobel seminar, Yamanaka cited the earlier seminal work of Harold Weintraub on the role of myoblast determination protein 1 (MyoD) in reprogramming cell fate to a muscle lineage as an important precursor to the discovery of iPSCs.
[10] iPSCs are typically derived by introducing products of specific sets of pluripotency-associated genes, or "reprogramming factors", into a given cell type.
[11] It is also clear that pro-mitotic factors such as C-MYC/L-MYC or repression of cell cycle checkpoints, such as p53, are conduits to creating a compliant cellular state for iPSC reprogramming.
Induced pluripotent stem cells were first generated by Shinya Yamanaka and Kazutoshi Takahashi at Kyoto University, Japan, in 2006.
[13][14][15][16] Reprogramming of human cells to iPSCs was reported in November 2007 by two independent research groups: Shinya Yamanaka of Kyoto University, Japan, who pioneered the original iPSC method, and James Thomson of University of Wisconsin-Madison who was the first to derive human embryonic stem cells.
[18] Obtaining fibroblasts to produce iPSCs involves a skin biopsy, and there has been a push towards identifying cell types that are more easily accessible.
The table on the right summarizes the key strategies and techniques used to develop iPS cells in the first five years after Yamanaka et al.'s 2006 breakthrough.
In 2008, Ding et al. used the inhibition of histone methyl transferase (HMT) with BIX-01294 in combination with the activation of calcium channels in the plasma membrane in order to increase reprogramming efficiency.
[43] Deng et al. of Beijing University reported in July 2013 that induced pluripotent stem cells can be created without any genetic modification.
Another key strategy for avoiding problems such as tumorgenesis and low throughput has been to use alternate forms of vectors: adenoviruses, plasmids, and naked DNA or protein compounds.
The adenovirus is unique from other vectors like viruses and retroviruses because it does not incorporate any of its own genes into the targeted host and avoids the potential for insertional mutagenesis.
Because non-retroviral approaches have demonstrated such low efficiency levels, researchers have attempted to effectively rescue the technique with what is known as the PiggyBac Transposon System.
Several studies have demonstrated that this system can effectively deliver the key reprogramming factors without leaving footprint mutations in the host cell genome.
[50] In light of difficulties that other labs had replicating the results of the surprising study, in March 2014, one of the co-authors has called for the articles to be retracted.
[51] On 4 June 2014, the lead author, Obokata agreed to retract both the papers[52] after she was found to have committed 'research misconduct' as concluded in an investigation by RIKEN on 1 April 2014.
For instance, iPS cell lines derived from patients affected by ectodermal dysplasia syndrome (EEC), in which the p63 gene is mutated, display abnormal epithelial commitment that could be partially rescued by a small compound.
This is particularly important because many other types of human cells derived from patients tend to stop growing after a few passages in laboratory culture.
[71][72] An international collaborated project, StemBANCC, was formed in 2012 to build a collection of iPS cell lines for drug screening for a variety of diseases.
The goal is to generate a library of 1,500 iPS cell lines which will be used in early drug testing by providing a simulated human disease environment.
[73] Furthermore, combining hiPSC technology and small molecule or genetically encoded voltage and calcium indicators provided a large-scale and high-throughput platform for cardiovascular drug safety screening.
[74][75][76][77][78] A proof-of-concept of using induced pluripotent stem cells (iPSCs) to generate human organ for transplantation was reported by researchers from Japan.
Further studies will monitor the longevity of the transplanted organ in the host body (ability to integrate or avoid rejection) and whether it will transform into tumors.
[93][94] In October 2019, a group at Okayama University developed a model of ischemic heart disease using cardiomyocytes differentiated from iPS cells.
[96] The first human clinical trial using autologous iPSCs was approved by the Japan Ministry Health and was to be conducted in 2014 at the Riken Center for Developmental Biology in Kobe.
[99][100] In March 2017, a team led by Masayo Takahashi completed the first successful transplant of iPS-derived retinal cells from a donor into the eye of a person with advanced macular degeneration.
[104] To make iPSC-based regenerative medicine technologies available to more patients, it is necessary to create universal iPSCs that can be transplanted independently of haplotypes of HLA.
[105] A multipotent mesenchymal stem cell, when induced into pluripotence, holds great promise to slow or reverse aging phenotypes.
[106] In 2020, Stanford University researchers concluded after studying elderly mice that old human cells when subjected to the Yamanaka factors, might rejuvenate and become nearly indistinguishable from their younger counterparts.