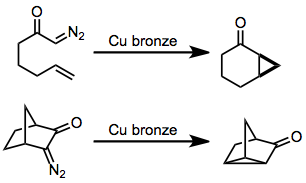
Intramolecular reactions of diazocarbonyl compounds
For enantioselective cyclopropanations and insertions, both copper- and rhodium-based catalysts are employed, although the latter have been more heavily studied in recent years.
In one example, intramolecular participation of an aryl group leads to the formation of a polycyclic ring system with complete diastereoselectivity.
[11] (4)α-Diazoesters are not as efficient as diazoketones at intramolecular cyclizations in some cases because of the propensity of esters to exist in the trans conformation about the carbon–oxygen single bond.
[12] However, intramolecular reactions of diazoesters do take place—in the example in equation (5), copper(II) sulfate is used to effect the formation of the cyclopropyl ester shown.
[6] (5)In the presence of a catalytic amount of acid, diazomethyl ketone substrates containing a pendant double bond or aryl group undergo cyclization.
The mechanism of this process most likely involves protonation of the diazocarbonyl group to form a diazonium salt, followed by displacement of nitrogen by the unsaturated functionality and deprotonation.
[17] Reactions mediated by copper are typically on the order of hours, and in some cases, slow addition of the diazocarbonyl compound is necessary.
The resulting orange oil was dissolved in benzene (2 x 5.0 mi, freshly distilled from calcium hydride) under nitrogen.
Tetrahydrofuran (40 ml, freshly distilled from lithium aluminum hydride) and finely divided metallic copper powder (0.67 g) were added to the crude diazo ketone, sequentially.
Fractions 11–16 gave 0.164 g (33%) of pure ketone product: mp 64-64.5° (from pentane); IR (CCl4) 3095 (cyclopropyl CH) and 1755 cm−1 (CO); NMR (CCl4) δ 1.18 (s, 3H, CH3) 1.03 (9, 3H, CH3), 0.97 (s, 3H, CH3), and 0.90 ppm (s, 3H, CH3).