Isopentenyl-diphosphate delta isomerase
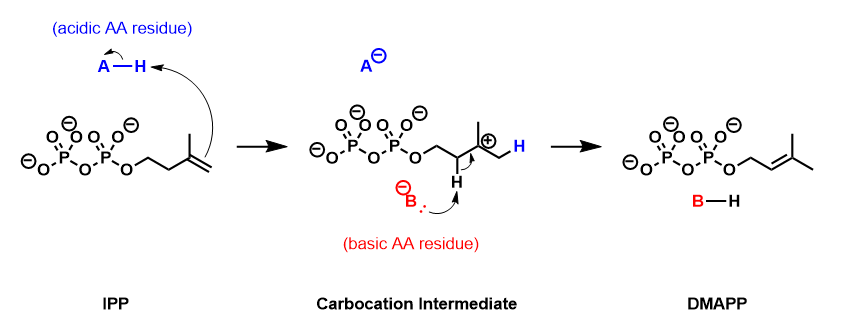
[5][6] The empirical evidence suggests that this reaction proceeds by a protonation/deprotonation mechanism, with the addition of a proton to the re-face of the inactivated C3-C4 double bond resulting in a transient carbocation intermediate.
The protonation of an inactivated double bond is rarely seen in nature, highlighting the unique catalytic mechanism of IPP isomerase.
The isomerization of IPP to DMAPP is a crucial step in the synthesis of isoprenoids and isoprenoid-derivatives, compounds that play vital roles in the biosynthetic pathways of all living organisms.
[15] Because of the importance of the melavonate pathway in isoprenoid biosynthesis, IPP isomerase is found in a variety of different cellular compartments, including plastids and mammalian mitochondria.
[16] Mutations in IDI1, the gene that codes for IPP isomerase 1, have been implicated in decreased viability in a number of organisms, including the yeast Saccharomyces cerevisiae, the nematode Caenorhabditis elegans and the plant Arabidopsis thaliana.