Kinetochore
[1] The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis.
Many of these proteins are conserved between eukaryotic species, including a specialized histone H3 variant (called CENP-A or CenH3) which helps the kinetochore associate with DNA.
Other proteins, such as Mad2, monitor the microtubule attachment as well as the tension between sister kinetochores and activate the spindle checkpoint to arrest the cell cycle when either of these is absent.
[9] On the other hand, microtubules are metastable polymers made of α- and β-tubulin, alternating between growing and shrinking phases, a phenomenon known as dynamic instability.
[10] MTs are highly dynamic structures, whose behavior is integrated with kinetochore function to control chromosome movement and segregation.
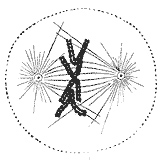
[11][12] The kinetochore is composed of several layers, observed initially by conventional fixation and staining methods of electron microscopy,[13][14] (reviewed by C. Rieder in 1982[15]) and more recently by rapid freezing and substitution.
The outermost domain in the kinetochore forms a fibrous corona, which can be visualized by conventional microscopy, yet only in the absence of MTs.
This corona is formed by a dynamic network of resident and temporary proteins implicated in the spindle checkpoint, in microtubule anchoring, and in the regulation of chromosome behavior.
Professor B. Nicklas (Duke University), showed that, if one breaks down the MT-kinetochore attachment using a laser beam, chromatids can no longer move, leading to an abnormal chromosome distribution.
This specificity guarantees that only one chromatid will move to each spindle side, thus ensuring the correct distribution of the genetic material.
Thus, one of the basic functions of the kinetochore is the MT attachment to the spindle, which is essential to correctly segregate sister chromatids.
To prevent this from happening, there are mechanisms of error detection and correction (as the spindle assembly checkpoint), whose components reside also on the kinetochores.
Another motor protein implicated in the initial capture of MTs is CENP-E; this is a high molecular weight kinesin associated with the fibrous corona at mammalian kinetochores from prometaphase until anaphase.
[53] In cells with low levels of CENP-E, chromosomes lack this protein at their kinetochores, which quite often are defective in their ability to congress at the metaphase plate.
[54] It is widely accepted that the kMTs fiber (the bundle of microtubules bound to the kinetochore) is originated by the capture of MTs polymerized at the centrosomes and spindle poles in mammalian cultured cells.
[55] The manner in which the centromeric region or kinetochore initiates the formation of kMTs and the frequency at which this happens are important questions,[according to whom?]
Mutants lacking any of the components of this complex show loss of the kinetochore-microtubule connection, although kinetochore structure is not completely lost.
[25][26][57][61][62][63][64] Studies on Hec1 (highly expressed in cancer cells 1), the human homolog of Ndc80p, show that it is important for correct chromosome congression and mitotic progression, and that it interacts with components of the cohesin and condensin complexes.
[65] Different laboratories have shown that the Ndc80 complex is essential for stabilization of the kinetochore-microtubule anchoring, required to support the centromeric tension implicated in the establishment of the correct chromosome congression in high eukaryotes.
[26][62][63][64] Cells with impaired function of Ndc80 (using RNAi, gene knockout, or antibody microinjection) have abnormally long spindles, lack of tension between sister kinetochores, chromosomes unable to congregate at the metaphase plate and few or any associated kMTs.
There is a variety of strong support for the ability of the Ndc80 complex to directly associate with microtubules and form the core conserved component of the kinetochore-microtubule interface.
During S-Phase, the cell duplicates all the genetic information stored in the chromosomes, in the process termed DNA replication.
Cells in which the function of this complex has been abolished by dominant negative mutants, RNAi, antibody microinjection or using selective drugs, accumulate errors in chromosome anchoring.
Many studies have shown that Aurora B is required to destabilize incorrect anchoring kinetochore-MT, favoring the generation of amphitelic connections.
The disappearance of the checkpoint proteins out of the kinetochores indicates the moment when the chromosome has reached the metaphase plate and is under bipolar tension.
[74] When Shugoshin levels are reduced by RNAi in HeLa cells, cohesin cannot remain on the centromeres during mitosis, and consequently sister chromatids separate synchronically before anaphase initiates, which triggers a long mitotic arrest.
This allows the kinetochores from cells at prometaphase to show "directional instability",[80] changing between persistent phases of movement towards the pole (poleward) or inversed (anti-poleward), which are coupled with alternating states of kMTs depolymerization and polymerization, respectively.