Lithium imide
Lithium imide is an inorganic compound with the chemical formula Li2NH.
[1] The product is light-sensitive and can undergo disproportionation to lithium amide and characteristically red lithium nitride.
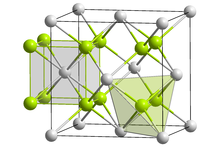
Lithium imide is thought to have a simple face-centered cubic structure with a Fm3m space group; with N-H bond distances of 0.82(6) Å and a H–N–H bond angle of 109.5°, giving it a similar structure to lithium amide.
[2][3] Lithium imide is strongly basic and deprotonates even some extremely weak acids such as methane and ammonia, due to the very localized negative charge on the nitrogen, which carries two formal charges.
It has been investigated as a material for hydrogen storage.