Hemoglobin
For example, the most common hemoglobin sequences in humans, bonobos and chimpanzees are completely identical, with exactly the same alpha and beta globin protein chains.
A mostly separate set of diseases called thalassemias involves underproduction of normal and sometimes abnormal hemoglobins, through problems and mutations in globin gene regulation.
Non-synonymous mutations in the hemoglobin gene of multiple species living at high elevations (Oreotrochilus, A. castelnaudii, C. violifer, P. gigas, and A. viridicuada) have caused the protein to have less of an affinity for inositol hexaphosphate (IHP), a molecule found in birds that has a similar role as 2,3-BPG in humans; this results in the ability to bind oxygen in lower partial pressures.
While the exact genotype and mechanism by which this occurs is not yet clear, selection is acting on these women's ability to bind oxygen in low partial pressures, which overall allows them to better sustain crucial metabolic processes.
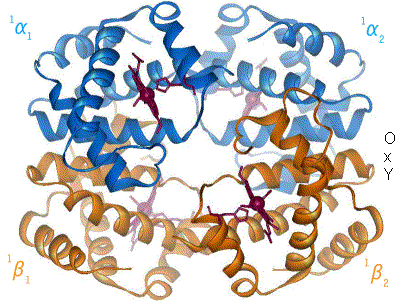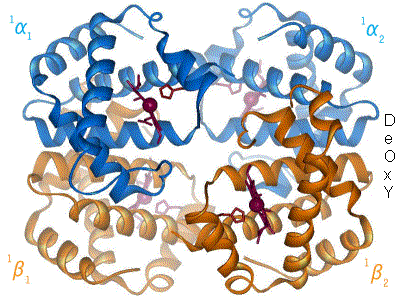
[citation needed] A heme group consists of an iron (Fe) ion held in a heterocyclic ring, known as a porphyrin.
This porphyrin ring consists of four pyrrole molecules cyclically linked together (by methine bridges) with the iron ion bound in the center.
[47] The iron ion, which is the site of oxygen binding, coordinates with the four nitrogen atoms in the center of the ring, which all lie in one plane.
[54] Oxyhemoglobin is formed during physiological respiration when oxygen binds to the heme component of the protein hemoglobin in red blood cells.
The oxygen then travels through the blood stream to be dropped off at cells where it is utilized as a terminal electron acceptor in the production of ATP by the process of oxidative phosphorylation.
[30] The predecessors of these genes arose through another duplication event also after the gnathosome common ancestor derived from jawless fish, approximately 450–500 million years ago.
[62] Ancestral reconstruction studies suggest that the preduplication ancestor of the α and β genes was a dimer made up of identical globin subunits, which then evolved to assemble into a tetrameric architecture after the duplication.
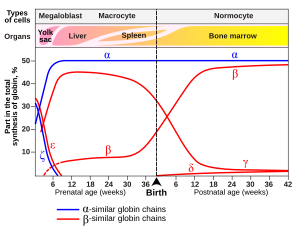
[65] These further duplications and divergences have created a diverse range of α- and β-like globin genes that are regulated so that certain forms occur at different stages of development.
This interaction forces the plane of the ring sideways toward the outside of the tetramer, and also induces a strain in the protein helix containing the histidine as it moves nearer to the iron atom.
[68] The binding of oxygen is affected by molecules such as carbon monoxide (for example, from tobacco smoking, exhaust gas, and incomplete combustion in furnaces).
When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue.
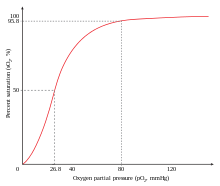
A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect.
[75] GTP reduces hemoglobin oxygen affinity much more than ATP, which is thought to be due to an extra hydrogen bond formed that further stabilizes the tense state.
[76] Under hypoxic conditions, the concentration of both ATP and GTP is reduced in fish red blood cells to increase oxygen affinity.
The resulting S-nitrosylated hemoglobin influences various NO-related activities such as the control of vascular resistance, blood pressure and respiration.
Iron is removed from heme and salvaged for later use, it is stored as hemosiderin or ferritin in tissues and transported in plasma by beta globulins as transferrins.
[87] The levels of glycated hemoglobin are therefore measured in order to monitor the long-term control of the chronic disease of type 2 diabetes mellitus (T2DM).
[89] Elevated levels of hemoglobin are associated with increased numbers or sizes of red blood cells, called polycythemia.
[91] This conversion factor, using the single globin unit molecular weight of 16,000 Da, is more common for hemoglobin concentration in blood.
[96] Laboratory hemoglobin test methods require a blood sample (arterial, venous, or capillary) and analysis on hematology analyzer and CO-oximeter.
[14] One of the most striking occurrences and uses of hemoglobin in organisms is in the giant tube worm (Riftia pachyptila, also called Vestimentifera), which can reach 2.4 meters length and populates ocean volcanic vents.
The worms' upper end is a deep-red fan-like structure ("plume"), which extends into the water and absorbs H2S and O2 for the bacteria, and CO2 for use as synthetic raw material similar to photosynthetic plants.
[12] It has been suggested that brain hemoglobin in these cells may enable the "storage of oxygen to provide a homeostatic mechanism in anoxic conditions, which is especially important for A9 DA neurons that have an elevated metabolism with a high requirement for energy production".
[12] It has been noted further that "A9 dopaminergic neurons may be at particular risk of anoxic degeneration since in addition to their high mitochondrial activity they are under intense oxidative stress caused by the production of hydrogen peroxide via autoxidation and/or monoamine oxidase (MAO)-mediated deamination of dopamine and the subsequent reaction of accessible ferrous iron to generate highly toxic hydroxyl radicals".
The intentional rusting of the initially shiny work of art mirrors hemoglobin's fundamental chemical reaction of oxygen binding to iron.
[113][114] Montreal artist Nicolas Baier created Lustre (Hémoglobine), a sculpture in stainless steel that shows the structure of the hemoglobin molecule.