Manganese(III) oxide
It occurs in nature as the mineral bixbyite (recently changed to bixbyite-(Mn)[3][4]) and is used in the production of ferrites and thermistors.
[5] Many preparations of nano-crystalline Mn2O3 have been reported, for example syntheses involving oxidation of MnII salts or reduction of MnO2.
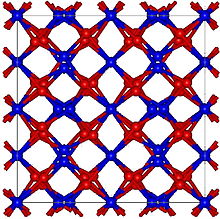
[9] Mn2O3 is unlike many other transition metal oxides in that it does not adopt the corundum (Al2O3) structure.
[11] α-Mn2O3 has the cubic bixbyite structure, which is an example of a C-type rare earth sesquioxide (Pearson symbol cI80, space group Ia3, #206).
[10] γ-Mn2O3 is ferrimagnetic with a Néel temperature of 39 K.[14] ε-Mn2O3 takes on a rhombohedral ilmenite structure (the first binary compound known to do so), wherein the manganese cations divided equally into oxidation states 2+ and 4+.