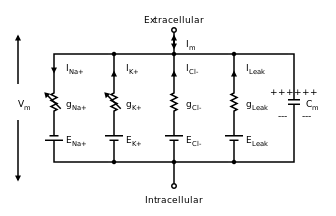
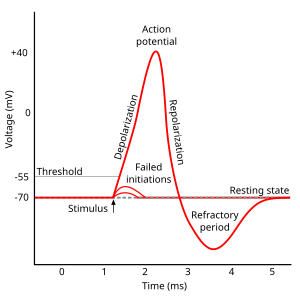
Action potential
[b] When the channels open, they allow an inward flow of sodium ions, which changes the electrochemical gradient, which in turn produces a further rise in the membrane potential towards zero.
Action potentials are driven by channel proteins whose configuration switches between closed and open states as a function of the voltage difference between the interior and exterior of the cell.
In the Hodgkin–Huxley membrane capacitance model, the speed of transmission of an action potential was undefined and it was assumed that adjacent areas became depolarized due to released ion interference with neighbouring channels.
[26][27][28][29] In sensory neurons, an external signal such as pressure, temperature, light, or sound is coupled with the opening and closing of ion channels, which in turn alter the ionic permeabilities of the membrane and its voltage.
For illustration, in the human ear, hair cells convert the incoming sound into the opening and closing of mechanically gated ion channels, which may cause neurotransmitter molecules to be released.
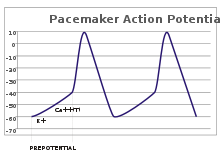
[g] Although such pacemaker potentials have a natural rhythm, it can be adjusted by external stimuli; for instance, heart rate can be altered by pharmaceuticals as well as signals from the sympathetic and parasympathetic nerves.
[7][10] A complicating factor is that a single ion channel may have multiple internal "gates" that respond to changes in Vm in opposite ways, or at different rates.
The voltages and currents of the action potential in all of its phases were modeled accurately by Alan Lloyd Hodgkin and Andrew Huxley in 1952,[i] for which they were awarded the Nobel Prize in Physiology or Medicine in 1963.
[lower-Greek 2] However, their model considers only two types of voltage-sensitive ion channels, and makes several assumptions about them, e.g., that their internal gates open and close independently of one another.
[43][44][45] At longer times, after some but not all of the ion channels have recovered, the axon can be stimulated to produce another action potential, but with a higher threshold, requiring a much stronger depolarization, e.g., to −30 mV.
[39] Combined, these changes in sodium and potassium permeability cause Vm to drop quickly, repolarizing the membrane and producing the "falling phase" of the action potential.
When closing after an action potential, sodium channels enter an "inactivated" state, in which they cannot be made to open regardless of the membrane potential—this gives rise to the absolute refractory period.
At the molecular level, this absolute refractory period corresponds to the time required for the voltage-activated sodium channels to recover from inactivation, i.e., to return to their closed state.
In order to enable fast and efficient transduction of electrical signals in the nervous system, certain neuronal axons are covered with myelin sheaths.
For axons larger than a minimum diameter (roughly 1 micrometre), myelination increases the conduction velocity of an action potential, typically tenfold.
[69] It is likely that the familiar signaling function of action potentials in some vascular plants (e.g. Mimosa pudica) arose independently from that in metazoan excitable cells.
[ap] Sponges seem to be the main phylum of multicellular eukaryotes that does not transmit action potentials, although some studies have suggested that these organisms have a form of electrical signaling, too.
The initial work, prior to 1955, was carried out primarily by Alan Lloyd Hodgkin and Andrew Fielding Huxley, who were, along John Carew Eccles, awarded the 1963 Nobel Prize in Physiology or Medicine for their contribution to the description of the ionic basis of nerve conduction.
[ar] These axons are so large in diameter (roughly 1 mm, or 100-fold larger than a typical neuron) that they can be seen with the naked eye, making them easy to extract and manipulate.
[av][aw] Refinements of this method are able to produce electrode tips that are as fine as 100 Å (10 nm), which also confers high input impedance.
[76] Action potentials may also be recorded with small metal electrodes placed just next to a neuron, with neurochips containing EOSFETs, or optically with dyes that are sensitive to Ca2+ or to voltage.
Optical imaging technologies have been developed in recent years to measure action potentials, either via simultaneous multisite recordings or with ultra-spatial resolution.
[bb] In the 19th century scientists studied the propagation of electrical signals in whole nerves (i.e., bundles of neurons) and demonstrated that nervous tissue was made up of cells, instead of an interconnected network of tubes (a reticulum).
Matteucci's work inspired the German physiologist, Emil du Bois-Reymond, who discovered in 1843 that stimulating these muscle and nerve preparations produced a notable diminution in their resting currents, making him the first researcher to identify the electrical nature of the action potential.
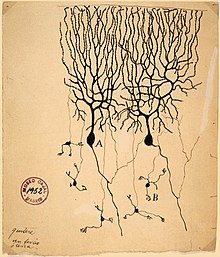
To establish that nervous tissue is made up of discrete cells, the Spanish physician Santiago Ramón y Cajal and his students used a stain developed by Camillo Golgi to reveal the myriad shapes of neurons, which they rendered painstakingly.
[bc][80] Bernstein's hypothesis was confirmed by Ken Cole and Howard Curtis, who showed that membrane conductance increases during an action potential.
[bd] In 1907, Louis Lapicque suggested that the action potential was generated as a threshold was crossed,[be] what would be later shown as a product of the dynamical systems of ionic conductances.
[i] Hodgkin and Huxley correlated the properties of their mathematical model with discrete ion channels that could exist in several different states, including "open", "closed", and "inactivated".
Their hypotheses were confirmed in the mid-1970s and 1980s by Erwin Neher and Bert Sakmann, who developed the technique of patch clamping to examine the conductance states of individual ion channels.
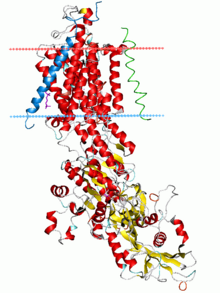
[h] The sodium–potassium pump was identified in 1957[bl][lower-Greek 6] and its properties gradually elucidated,[bm][bn][bo] culminating in the determination of its atomic-resolution structure by X-ray crystallography.



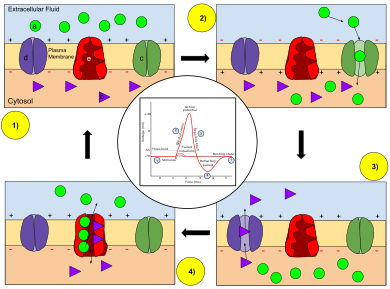
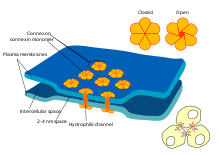
Key: a) Sodium (Na + ) ion. b) Potassium (K + ) ion. c) Sodium channel. d) Potassium channel. e) Sodium-potassium pump.
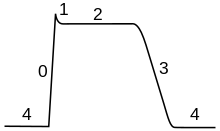
In the stages of an action potential, the permeability of the membrane of the neuron changes. At the resting state (1), sodium and potassium ions have limited ability to pass through the membrane, and the neuron has a net negative charge inside. Once the action potential is triggered, the depolarization (2) of the neuron activates sodium channels, allowing sodium ions to pass through the cell membrane into the cell, resulting in a net positive charge in the neuron relative to the extracellular fluid. After the action potential peak is reached, the neuron begins repolarization (3), where the sodium channels close and potassium channels open, allowing potassium ions to cross the membrane into the extracellular fluid, returning the membrane potential to a negative value. Finally, there is a refractory period (4), during which the voltage-dependent ion channels are inactivated while the Na + and K + ions return to their resting state distributions across the membrane (1), and the neuron is ready to repeat the process for the next action potential.