Organocopper chemistry
With 10 electrons in its valence shell, the bonding behavior of Cu(I) is similar to Ni(0), but owing to its higher oxidation state, it engages in less pi-backbonding.
Organic derivatives of copper's higher oxidation states of +2 and +3 are sometimes encountered as reaction intermediates, but rarely isolated or even observed.
Organocopper compounds form complexes with a variety of soft ligands such as alkylphosphines (R3P), thioethers (R2S), and cyanide (CN−).
Due to the spherical electronic shell of Cu+, copper(I) complexes have symmetrical structures - either linear, trigonal planar or tetrahedral, depending on the number of ligands.
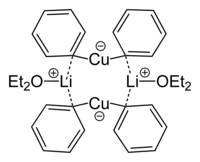
Alternatively, these cuprates are prepared from oligomeric neutral organocopper compounds by treatment with one equivalent of organolithium reagent.
The electronegativity of copper is much higher than its next-door neighbor in the group 12 elements, zinc, suggesting diminished nucleophilicity for its carbon ligands.
On the other hand, reactions of organocopper compound with alkenyl halides proceed with retention of subtrate’s configuration.
Aggregation is especially evident for charge-neutral organocopper compounds, i.e. species with the empirical formula (RCu), which adopt cyclic structures.
A cyclic structure is also seen for CuCH2SiMe3, where Me stands for methyl group CH3, the first 1:1 organocopper compound to be analyzed by X-ray crystallography (1972 by Lappert).
[10] The involvement of the otherwise rare Cu(III) oxidation state has been demonstrated in the conjugate addition of the Gilman reagent to an enone:[11] In a so-called rapid-injection NMR experiment at −100 °C, the Gilman reagent Li+[Cu(CH3)2]− (stabilized by lithium iodide) was introduced to cyclohexenone (1) enabling the detection of the copper — alkene pi complex 2.
With other ligands than the cyano group this study predicts room temperature stable Cu(III) compounds.
The approximate order of reactivity, beginning with the most reactive, is as follows: acid chlorides[14] > aldehydes > tosylates ~ epoxides > iodides > bromides > chlorides > ketones > esters > nitriles >> alkenes Generally the OA-RE mechanism is analogous to that of palladium-catalyzed cross coupling reactions.
Carbocupration is a nucleophilic addition of organocopper reagents (R−Cu) to acetylene or terminal alkynes resulting in an alkenylcopper compound (R2C=C(R)−Cu).
This method was proved to be very effective for the oxidative coupling of amines and alkyl, including tert-butyl, and aryl halides.
Muller and collaborators reported a vicinal functionalization of α,β-acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in the figure above) carbocupration favors the formation of the Z-aldol.