Organotitanium chemistry
[2] Independently, titanium-based Ziegler–Natta catalysts were described leading to major commercial applications, for which the 1963 Nobel Prize in Chemistry was awarded.
Simple tetraalkyltitanium compounds however are not typically isolable, owing to the large size of titanium and the electron-deficient nature of its tetrahedral complexes.
More abundant and more useful than the simple tetraalkyl compounds are mixed ligand complexes with alkoxide and cyclopentadienyl coligands.
Compounds of titanium in the +2 oxidation state are rarer, examples being titanocene dicarbonyl and Ti(CH3)2(dmpe)2.
On the other hand, high oxophilicity means that titanium alkyls are effective for abstracting or exchanging organyl ligands for oxo groups, as discussed below.
Methyltitanium trichloride, nominally CH3TiCl3, can be prepared by treating titanium(IV) chloride with dimethylzinc in dichloromethane at −78 °C.
[9] A dialkyltitanium species is implicated for Ti-promoted cyclopropanations starting from a Grignard reagent and an ester.
It can for example also be applied in a conversion of a ketene into an allene:[8][13] Attempted synthesis of "titanocene", i.e. Ti(C5H5)2, produces a fulvalene complex.
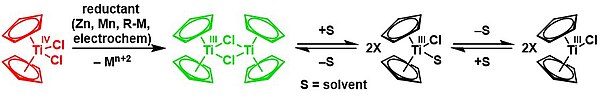
[14][16] The titanocene dimer was recognised in the 1970s[16][17][18] but not structurally characterised until 1992,[15] and the investigations led to many innovations on cyclopentadienyl complexes of titanium.
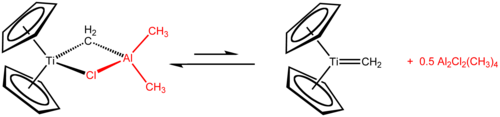
Tebbe's reagent adds simple alkenes to give titanocyclobutanes, which can be regarded as stable olefin metathesis intermediates.