Transition metal oxo complex
One of the earliest synthetic compounds to incorporate an oxo ligand is potassium ferrate (K2FeO4), which was likely prepared by Georg E. Stahl in 1702.
[2] A common reaction exhibited by metal-oxo compounds is olation, the condensation process that converts low molecular weight oxides to polymers with M-O-M linkages.
[4] Some of the longest known and most widely used oxo compounds are oxidizing agents such as potassium permanganate (KMnO4) and osmium tetroxide (OsO4).
[5] Compounds such as these are widely used for converting alkenes to vicinal diols and alcohols to ketones or carboxylic acids.
Although there is some variation among these enzymes, members from all three families involve oxygen atom transfer between the Mo/W center and the substrate.
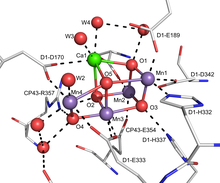
[16] The active site for the oxygen-evolving complex (OEC) of photosystem II (PSII) is a Mn4O5Ca centre with several bridging oxo ligands that participate in the oxidation of water to molecular oxygen.
[17] The OEC is proposed to utilize a terminal oxo intermediate as a part of the water oxidation reaction.
[18] Terminal oxo ligands are also rather rare for the titanium triad, especially zirconium and hafnium and are unknown for group 3 metals (scandium, yttrium, and lanthanum).